Part one of this topic was posted more than ten years ago.[1] I clearly forgot about it, so belatedly, here is part 2 – dealing with the stereochemistry of the reduction of tert-butyl-cyclohexanone by borohydride in water. The known stereochemistry is nicely summarised in this article, along with an extensive history of possible explanations of the reasons for the stereochemical preference.[2] Put simply, the hydride nucleophile attacks the carbonyl from an axial rather than equation direction with a ratio of 10:1 (ΔΔG 1.37 kcal/mol). So does the model I previously proposed[1] support this and give any indication of why the stereochemistry is axial?
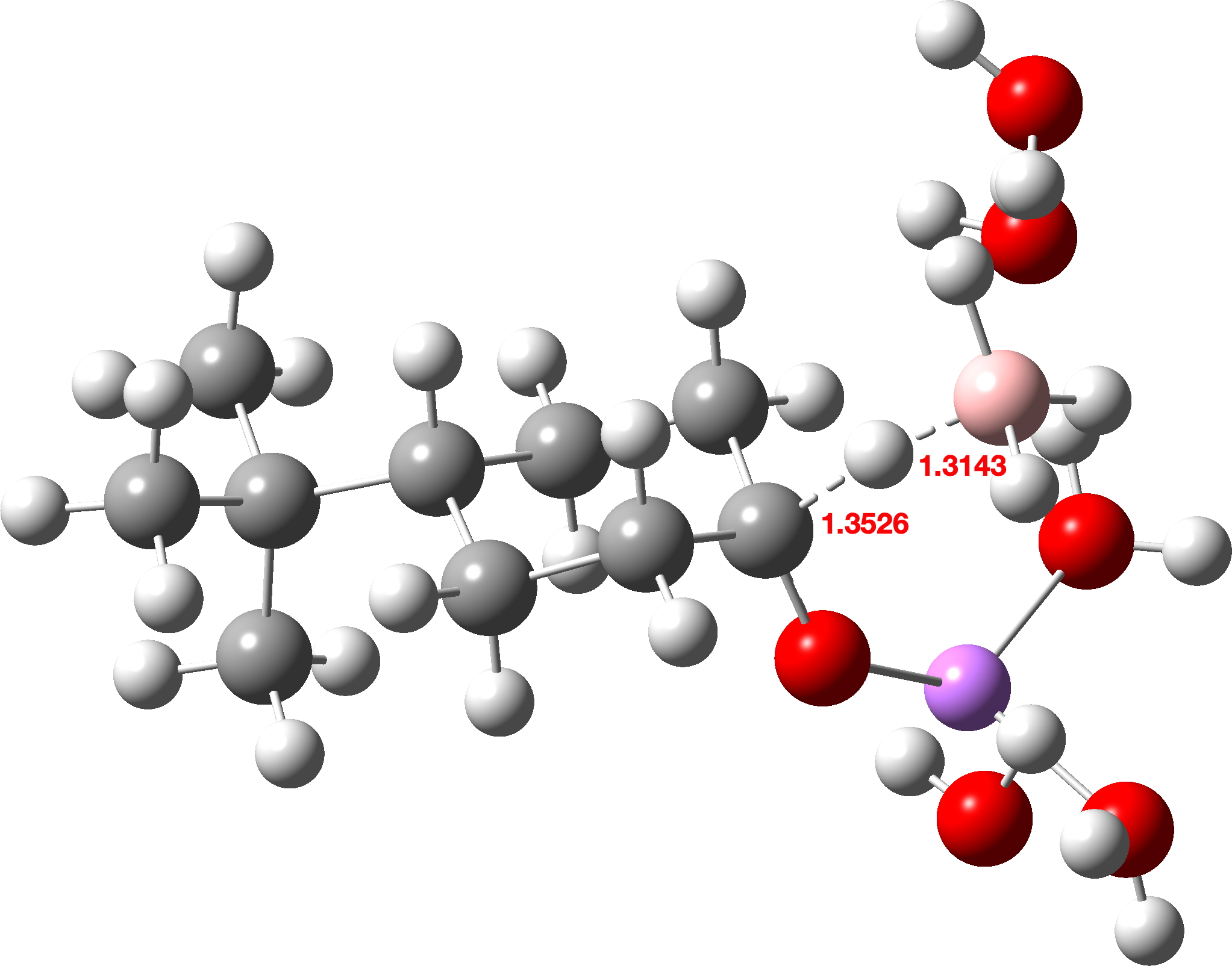
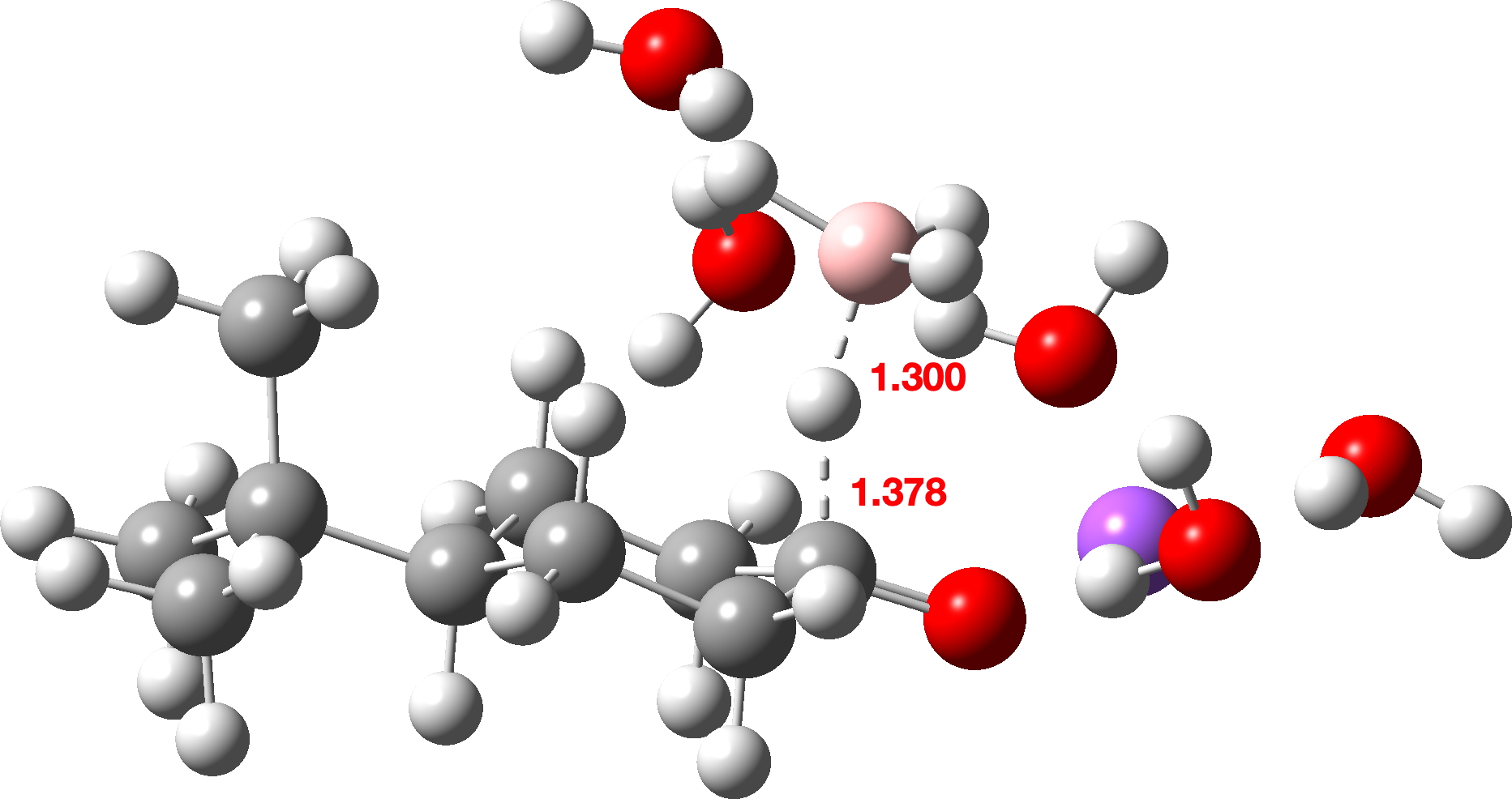
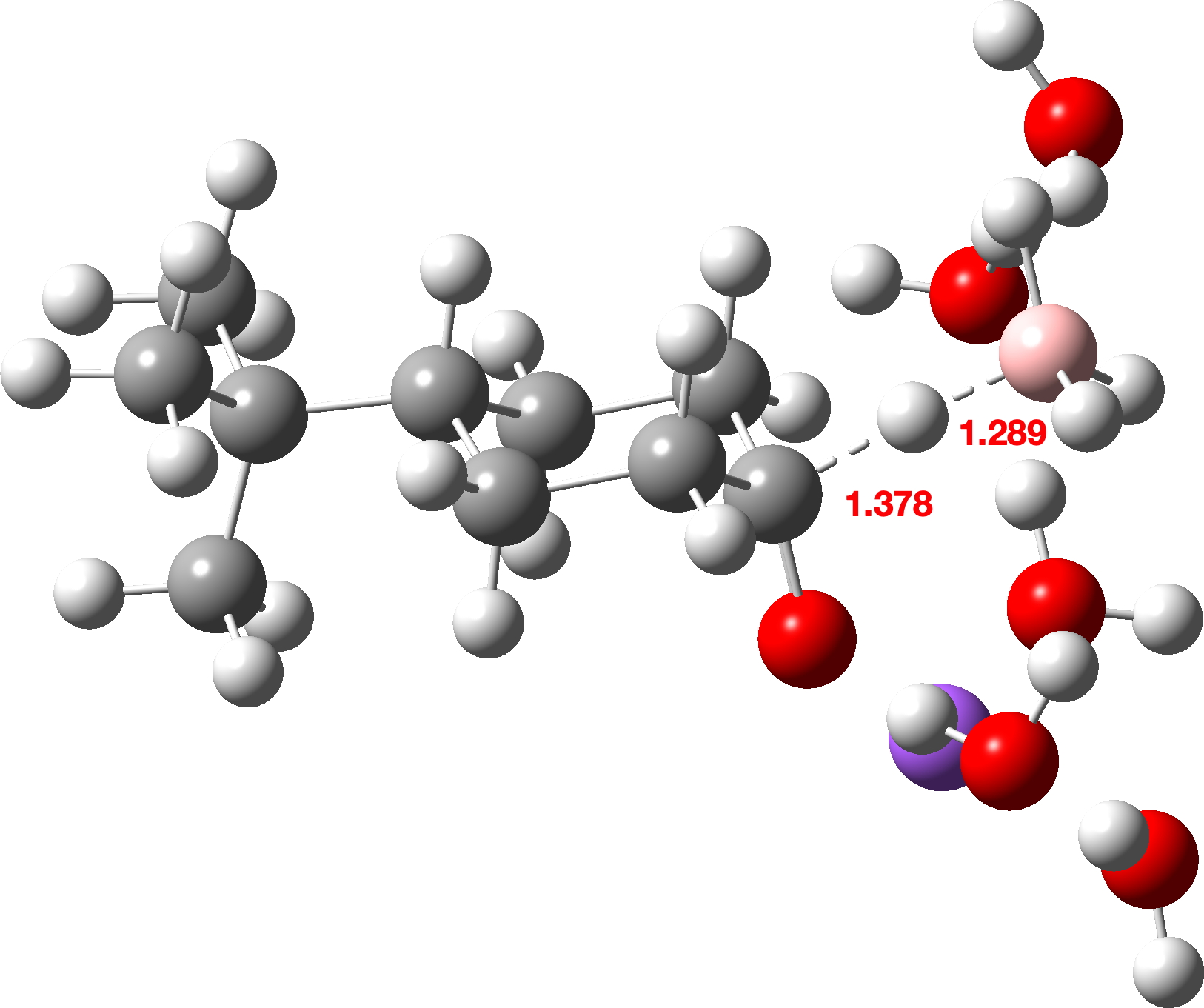
The calculated transition states are shown below (click on image to get interactive 3D model). Note also the unusual calculated B-H…H-O hydrogen bonded distances of ~1.8Å. A search of the CSD (crystal structure database) reveals surprisingly few such examples, but one interesting one is as short as ~1.73Å[3]
DFT calculations were conducted using Gaussian 16 at the B3LYP/Def2-TZVPP/SCRF=water level[4] for transition state location and energies (including D3+BJ dispersion) and using ORCA 6.1[5] for calculating D4 dispersion energies.[6]
Lithium Borohydride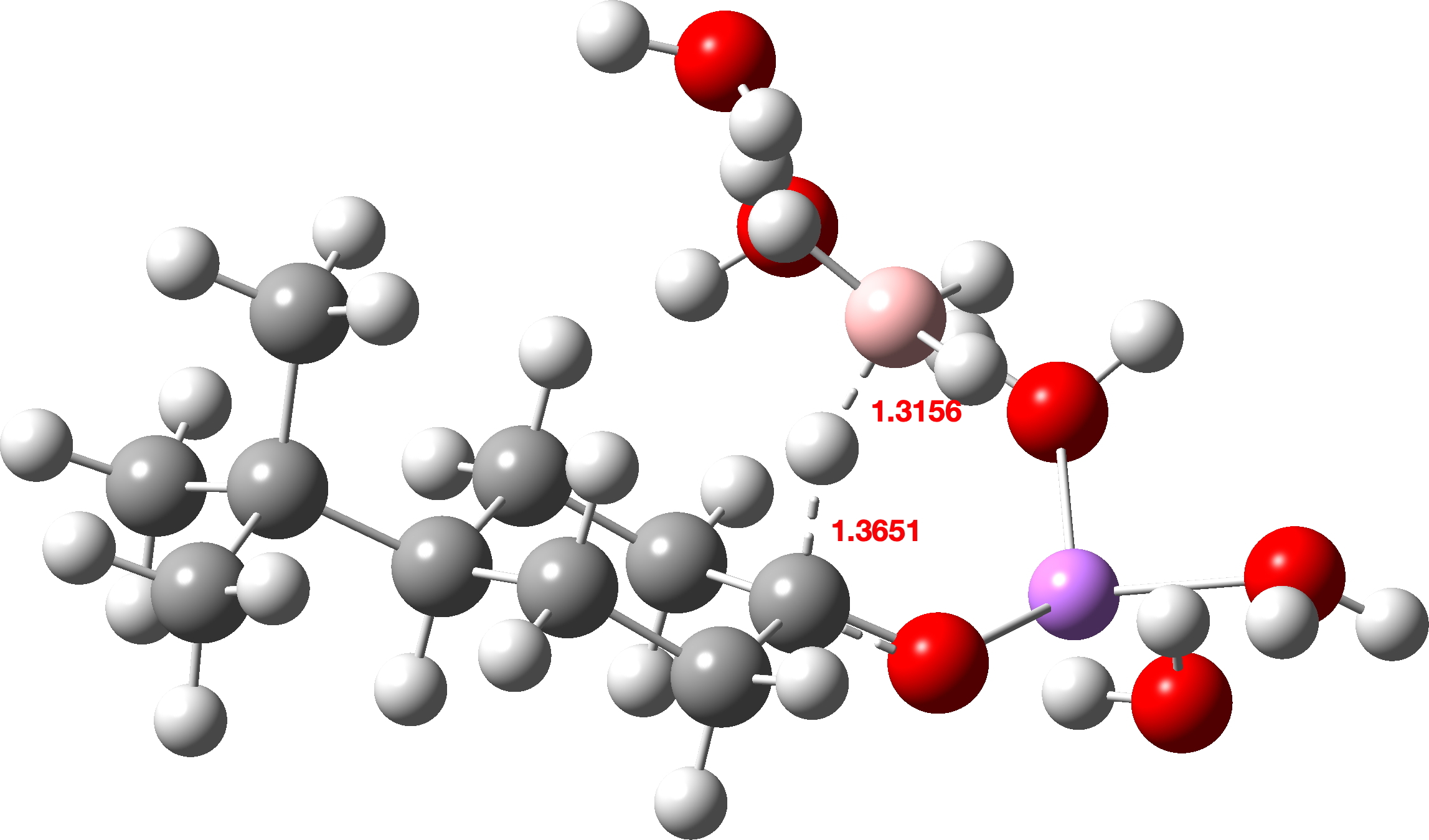
Sodium borohydride
As well as computing the free energy difference ΔΔG‡ between the two transition states, I also looked at the total dispersion energy contributions to these energies at the third generation D3+BJ and the fourth generation D4 levels, shown below as the difference between the axial and equatorial transition states.
| Counter ion | ΔD3 dispersion | ΔD4 dispersion | ΔG‡axial | ΔG‡equatorial | ΔΔGh† |
|---|---|---|---|---|---|
| Lithium borohydride | 1.15 | 0.932 | 12.13 | 12.93 | 0.80 |
| Sodium borohydride | 1.22 | 0.745 | 13.31 | 14.16 | 0.85 |
†Harmonic energies.
The calculations reveal that using either Li or K as the counterion to borohydride, the axial transition state is lower in energy than the equatorial, by around 0.85 to 0.80 kcal/mol in total free energy. These values correspond to an axial/equatorial ratio of around 3.9-4.2:1, a little bit lower than observed experimental value of 10:1.[2] The D3+BJ and D4 dispersion energies follow the same trend, with the suggestion that the Li ion is slightly more stereoselective than the Na ion, perhaps due to the more compact nature of the transition state.
So we might conclude that the stereochemical preference for axial hydride delivery to tert-butyl-cyclohexanone could be explained entirely by the differing dispersion energy contributions of the two transition states. This in one way is a deeply unsatisfactory explanation, since the dispersion energy difference is the total sum of many individual dispersion contributions and is largely unpredictable until calculated. Chemists like simple rules and this is not apparently amenable to such a simple rule. Indeed perhaps the rule should simply be to always compare the computed dispersion energies of two (or more) isomeric transition states before seeking other explanations!
A source of error in the calculated free energies could be the treatment of the vibrational modes as harmonic. A quasi-harmonic treatment is available[7] which should give a more reliable estimate of the relative free energy. Using this approach (for settings see [8]) ones gets the values below. Although the discrimination is slightly reduced, the overall prediction of axial hydride attack is still confirmed.
| system> | hG | qhG |
|---|---|---|
| LiBH4 ax | -884.325907 | -884.328233 (ΔΔG -0.62) |
| LiBH4 eq | -884.324625 | -884.327246 |
| NaBH4 ax | -1039.096789 | -1039.100009 (ΔΔG -0.78) |
| NaBH4 eq | -1039.095445 | -1039.098771 |
References
- H. Rzepa, “Part 1: ethanal.”, 2015.
- R. Kobetić, V. Petrović-Peroković, V. Ključarić, B. Juršić, and D.E. Sunko, “Selective Reduction of Cyclohexanones with NaBH4 in β-Cyclodextrin, PEG-400, and Micelles”, Supramolecular Chemistry, vol. 20, pp. 379-385, 2008.
- R. Custelcean, and J.E. Jackson, “Topochemical Control of Covalent Bond Formation by Dihydrogen Bonding”, Journal of the American Chemical Society, vol. 120, pp. 12935-12941, 1998.
- H. Rzepa, “The mechanism of borohydride reductions. Part 2: 4-t-butyl-cyclohexanone”, 2025.
- F. Neese, “Software Update: The
ORCA Program System—Version 6.0″, WIREs Computational Molecular Science, vol. 15, 2025. - E. Caldeweyher, J. Mewes, S. Ehlert, and S. Grimme, “Extension and evaluation of the D4 London-dispersion model for periodic systems”, Physical Chemistry Chemical Physics, vol. 22, pp. 8499-8512, 2020.
- G. Luchini, J.V. Alegre-Requena, I. Funes-Ardoiz, and R.S. Paton, “GoodVibes: automated thermochemistry for heterogeneous computational chemistry data”, F1000Research, vol. 9, pp. 291, 2020.
- H. Rzepa, “[Embargoed]”, 2025.
Related
You can leave a response, or trackback from your own site.
