Previously, I pondered about the strange N=N double bond in nitrosobenzene dimer[1] as a follow up to commenting on the curly arrow mechanism of the dimerisation.[2] By the same curly arrow method, one can produce the below, showing how the simpler nitric oxide radical could potentially dimerise to a species with a N≡N triple bond!† This involves a total of six electrons entering the N-N region, and hence raises the question of whether these all move in a single concerted/synchronous bond forming reaction, or whether they might go in (asynchronous) stages. Here are some calculations[3]) which might shed some light on this aspect.
The structure[4] of a nitric oxide dimer was shown in 1982 to have a very long (rather than short) N-N bond length of 2.237Å and a theoretical analysis[5] showed it to be a weak complex with a very complex wavefunction showing multi-reference character.
Firstly, an IRC-based reaction path (method: uωB97XD, scrf=(cpcm,solvent=water) guess=(mix,always) def2tzvpp to allow either an open shell biradical to form and also to encourage any ion pair formation). As you can see, the (total) energy goes up to a very shallow transition state (with a tiny reverse barrier) to form a biradical with 2> 0.628. This species, as noted existing in a very shallow energy well, has an N-N bond length of 1.725Å.
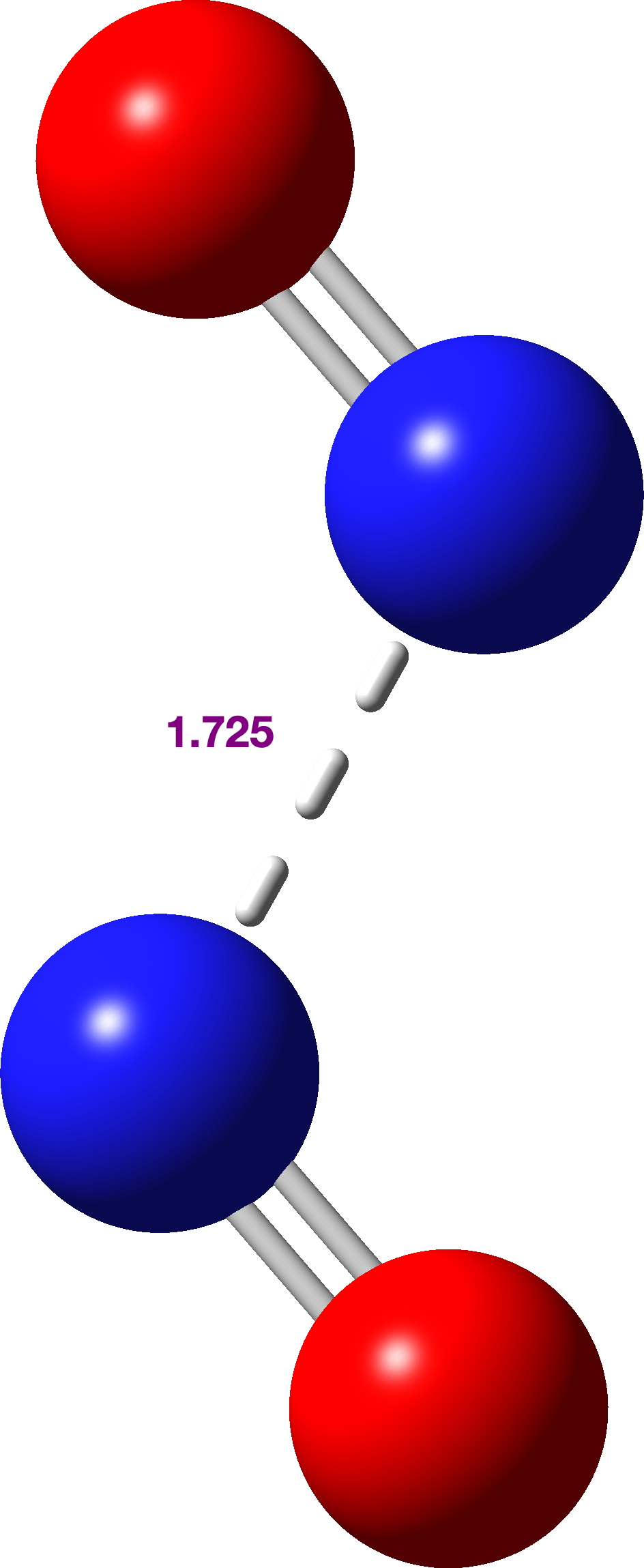
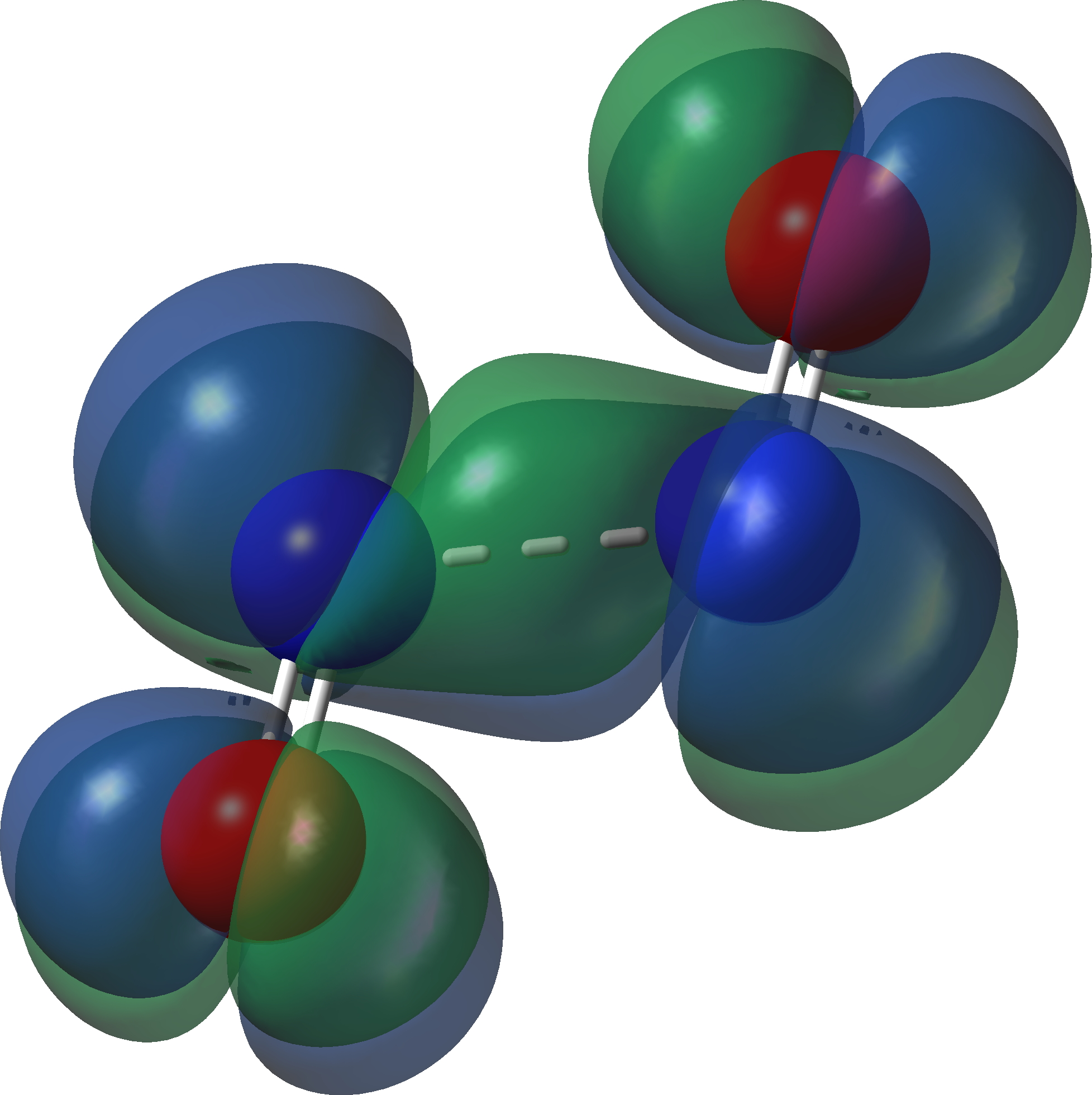
The bonding for this species is complex (analysis for a later post), but the calculated biradical spin density below shows the unpaired electrons are in the π-system (click on the image to get a 3D rotatable model).
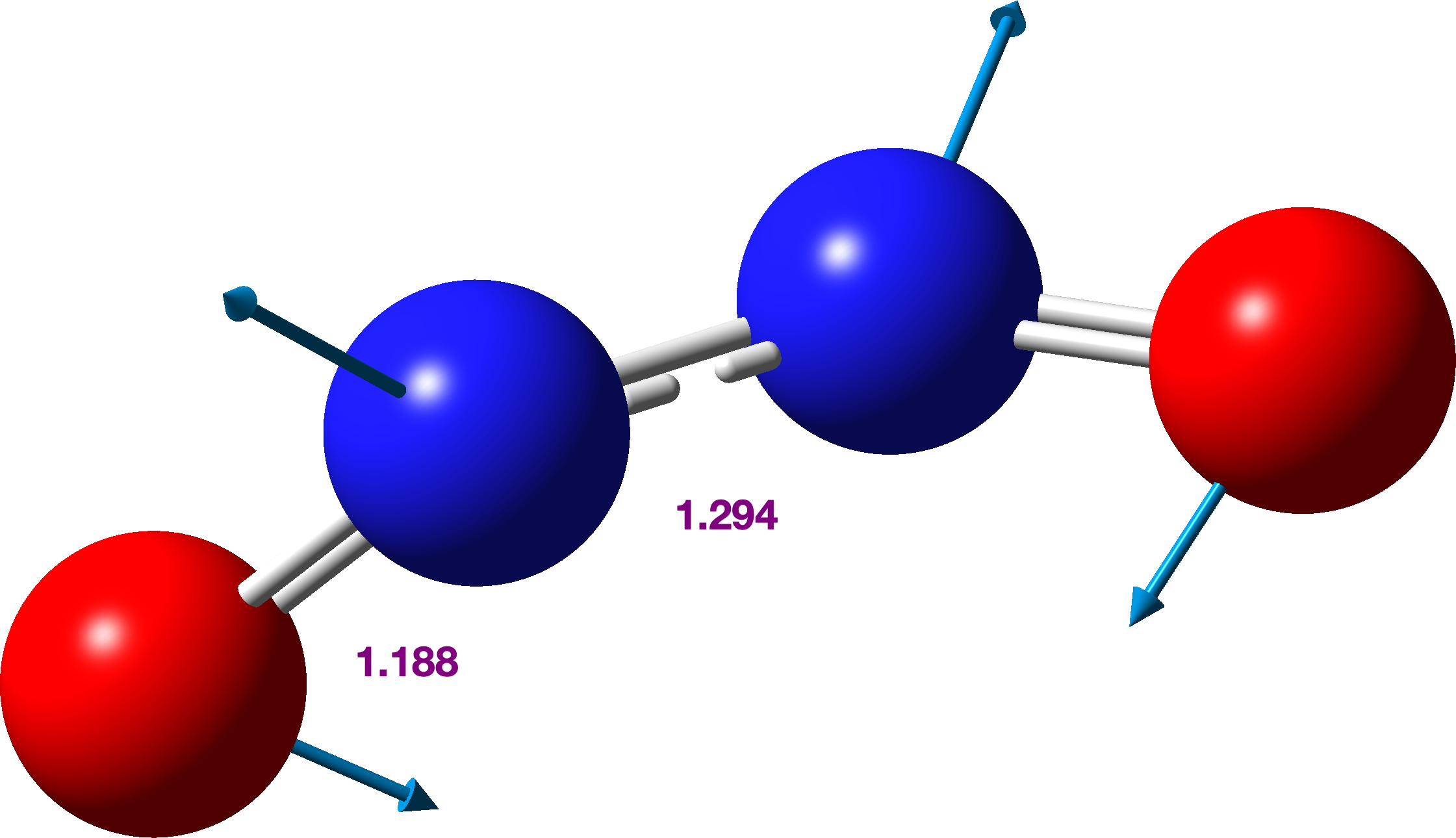
Further contraction of the N-N length results in an IRC energy potential to a transition state with a N-N length 1.294Å across a further barrier of ~12 kcal/mol (ΔE; ΔG 13.6 kcal/mol). The overall barrier from two nitric oxide molecules is ΔG 31.0 kcal/mol with the overall thermochemistry summarised in the table. Basically, this barrier is unsurmountable at normal temperatures and the reverse barrier of ΔG 6.7 kcal/mol ensures that the N≡N triple bonded species shown above is not likely stable and will not be observed experimentally.‡ However this product is NOT a biradical but a normal closed shell singlet molecule.[6]
So to answer my first question, the six electrons appear to move in two stages, firstly two electrons form a weak N-N bond and then a further four electrons contract this to a triple bond. Their motion is effectively concerted, but asynchronous.
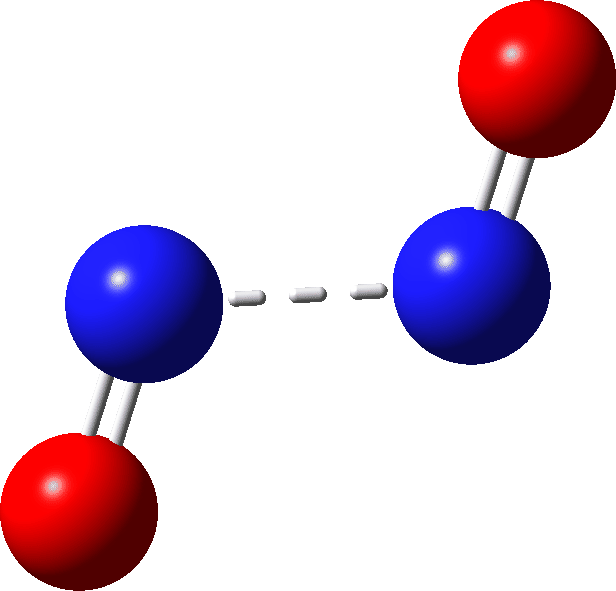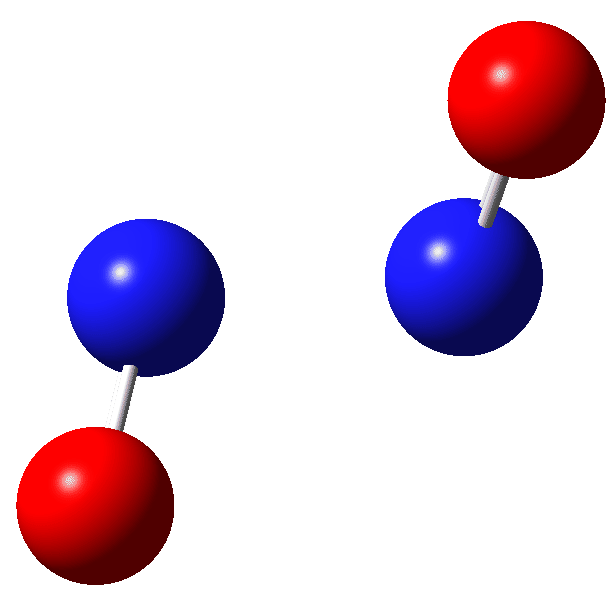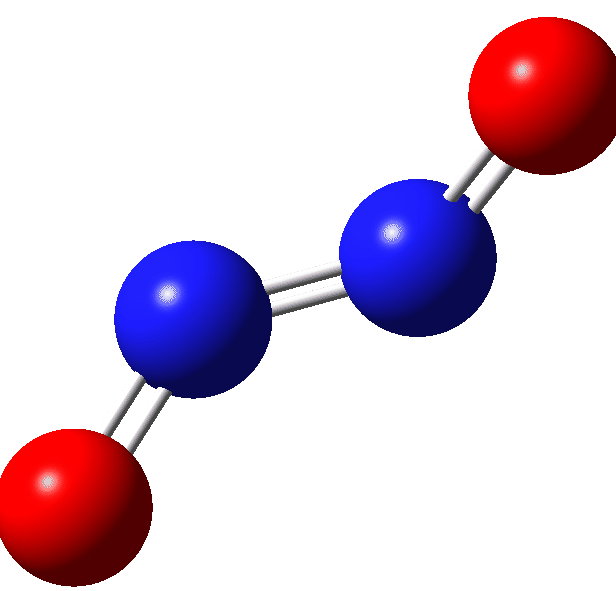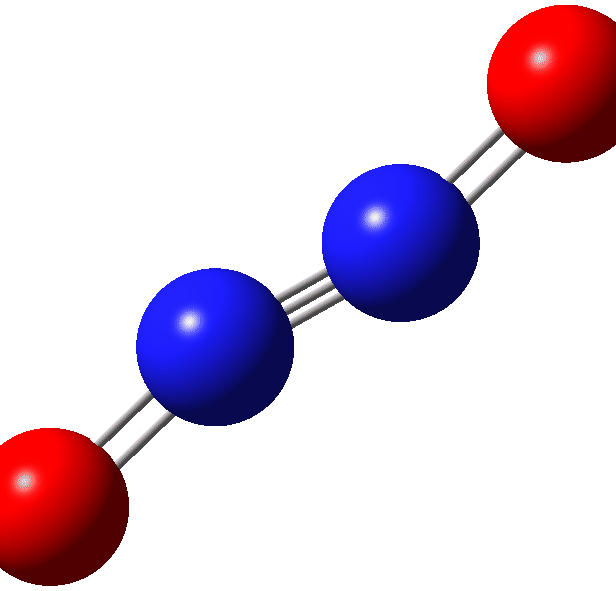
| Species | ΔG | ΔH | ΔΔG | ΔΔH | T.ΔS | rNN, Å | DOI | |
|---|---|---|---|---|---|---|---|---|
| 2*Nitric oxide | -259.83494 | -259.78839 | 0.0 | 0.0 | 29.2 | ∞ | 0.753 | 15472[7] |
| Singlet biradical | -259.80716 | -259.77615 | 17.4 | 7.7 | 19.5 | 1.725 | 0.628 | 15476[8] |
| Triplet biradical | -259.80865 | -259.77672 | 16.5 | 7.3 | 20.0 | 1.779 | 2.016 | 15475[9] |
| Singlet TS | -259.78550 | -259.75579 | 31.0 (13.6) | 20.5 (12.8) | 18.6 | 1.294 | 0.000 | 15483[3] |
| Singlet N≡N dimer | -259.79614 | -259.76693 | 24.3 (7.8) | 13.5 | 18.3 | 1.114 | 0.000 | 15467[10] |
Now for a NEDA energy decomposition analysis[11]
Electrical (ES+POL+SE) : -9414.608
Charge Transfer (CT) : -1363.597
Core (XC+DEF-SE) : 10782.725
Total Interaction (E) : 4.520 kcal/mol.
Normally NEDA total interaction energies are -ve, but this one is positive! So the triple bond dissociation energy is not merely small, but actually negative. That is a weak triple bond and as the title implies, a very mysterious bond. In some aspects however it is conventional. Thus calculated rNN 1.114Å and νNN 2604 cm-1. However partial occupancies of NBO antibonding BD* orbitals results in a calculated Wiberg bond order of only 1.01; there is still a great deal of mystery left about this species! Probably what is fairly certain is that the closed shell single-reference wavefunction used here is not appropriate for a full explanation and more complex multi-reference procedures would have to be used to get a more complete picture of this strange non-existing‡ little molecule. It may even be that such procedures remove the small reverse barrier noted above, thus preventing the molecule from even existing in an energy well.
†This species does not appear to have been previously discussed or suggested, according to SciFinder/CAS.
‡Might it exist at very high pressures in water?
To find all blog posts authored here, along with their DOIs, try
References
- H. Rzepa, “The mysterious N=N double bond in nitrosobenzene dimer.”, 2025.
- H. Rzepa, “Mechanism of the dimerisation of Nitrosobenzene.”, 2025.
- H. Rzepa, “N2O2 as strong dimer TS as biradical cis, G = -259.785500”, 2025.
- S.G. Kukolich, “The structure of the nitric oxide dimer”, Journal of the American Chemical Society, vol. 104, pp. 4715-4716, 1982.
- N. Taguchi, Y. Mochizuki, T. Ishikawa, and K. Tanaka, “Multi-reference calculations of nitric oxide dimer”, Chemical Physics Letters, vol. 451, pp. 31-36, 2008.
- H. Rzepa, “N2O2 as strong dimer? G = -259.796140, STABLE wavefunction!”, 2025.
- H. Rzepa, “Nitric oxide monomer, G = -129.917471 *2 = -259.834942”, 2025.
- H. Rzepa, “N2O2 as strong dimer singlet trans biradical state G = -259.807165”, 2025.
- H. Rzepa, “N2O2 as strong dimer triplet state G = -259.808649 DG 16.5”, 2025.
- H. Rzepa, “N2O2 as strong dimer? bent G = -259.796140”, 2025.
- E.D. Glendening, and A. Streitwieser, “Natural energy decomposition analysis: An energy partitioning procedure for molecular interactions with application to weak hydrogen bonding, strong ionic, and moderate donor–acceptor interactions”, The Journal of Chemical Physics, vol. 100, pp. 2900-2909, 1994.
This entry was posted on Monday, August 18th, 2025 at 10:50 am and is filed under Interesting chemistry. You can follow any responses to this entry through the RSS 2.0 feed.
You can leave a response, or trackback from your own site.

4 Comments
https://shorturl.fm/oSfwQ
https://shorturl.fm/RaUJi
zsqwg8
iefiij