Structural characterization
The crystal structures of the (2E)−1,4-bis(4-halophenyl)but-2-ene-1,4-dione compounds (C16H10 × 2O2, where X = F, Cl, Br, I) were investigated using first-principles calculations based on density functional theory (DFT). These chalcone-like derivatives crystallize in the monoclinic space group P2₁/c (No. 14) with Z = 2 formula units per unit cell. Figure 1; shows the molecular structure of the brominated compound (C16H10Br2O2) as a representative example of this family. The compounds C₁₆H₁₀X₂O₂ (X = F, Cl, Br, I) represent a series of halogenated derivatives whose crystal structures are notably influenced by the type of halogen. The systematic variation in the unit cell parameters correlates with the increasing atomic radius and decreasing electronegativity of the halogen atoms when moving from F to I. The optimized lattice parameters for all four compounds are summarized in Table 1,
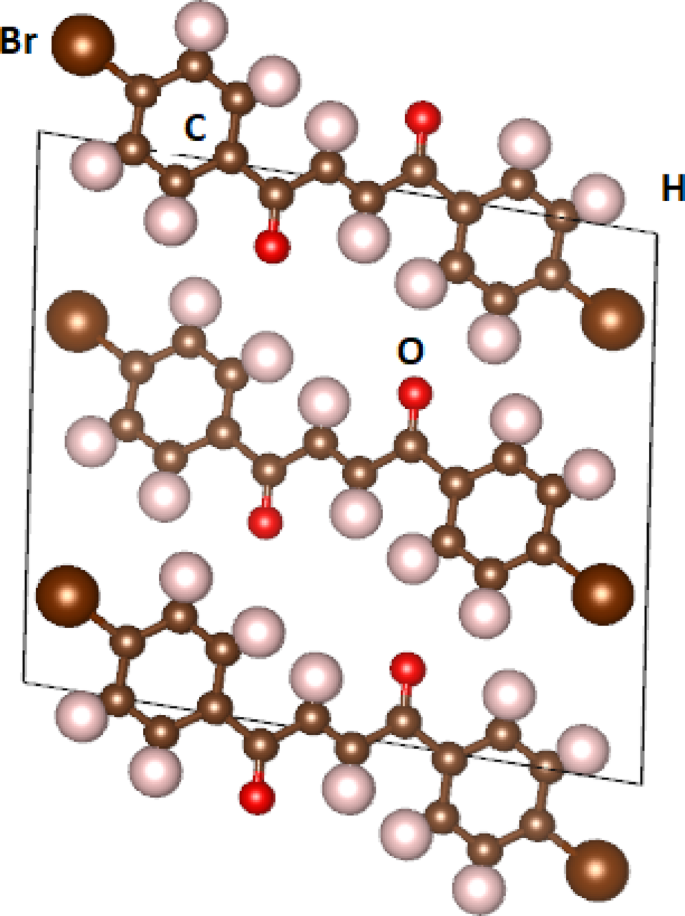
Crystal structures of the (2E)−1,4-bis(4-halophenyl)but-2-ene-1,4-dione compounds (C16H10Br2O2).
The calculated data show a decrease in the lattice parameter a and an increase in c as the halogen atom becomes larger, indicating elongation along the c-axis. The ratios c/a and c/b also increase progressively from F to I. Among the studied derivatives, both the chlorinated and brominated compounds (C₁₆H₁₀Cl₂O₂ and C₁₆H₁₀Br₂O₂) were experimentally characterized by single-crystal X-ray diffraction22, allowing direct comparison with the DFT-optimized structures. For the Cl compound, the experimental unit cell parameters are a = 13.95 Å, b = 6.08 Å, c = 14.68 Å, while the calculated values are a = 12.66 Å, b = 6.16 Å, c = 13.48 Å. For the Br compound, experimental parameters are a = 14.44 Å, b = 5.99 Å, c = 12.72 Å (monoclinic), compared to the computed ones: a = 12.39 Å, b = 6.21 Å, c = 13.57 Å. While some deviations exist—particularly in the b-axis—the calculated values generally reproduce the experimental trends in lattice expansion and symmetry. or the fluorinated and iodinated derivatives (C₁₆H₁₀F₂O₂ and C₁₆H₁₀I₂O₂), only theoretical data are currently available. However, based on the good agreement observed between DFT-calculated and experimentally determined lattice parameters for the chlorinated and brominated compounds, it is reasonable to assume that the predicted structures for the remaining derivatives are reliable and can serve as a solid reference for future experimental validation. The optimized lattice parameters for C₁₆H₁₀F₂O₂ are a = 13.62 Å, b = 6.90 Å, c = 14.33 Å, while those for C₁₆H₁₀I₂O₂ are a = 12.54 Å, b = 6.19 Å, c = 13.73 Å. These values reflect the expected structural trends across the halogen series, further supporting the consistency and predictive power of the DFT approach used in this study.
Electronic properties
Electronic structure
The electronic band structures of the compounds C₁₆H₁₀X₂O₂ (where X = F, Cl, Br, I) were calculated using DFT methods are show in Fig. 2, with the Fermi level set at 0 eV. These band structure diagrams provide insight into the effect of halogen substitution on the electronic properties of the studied molecules.In the band structure of C₁₆H₁₀Br₂O₂, a clear energy gap is observed between the valence and conduction bands, indicating semiconducting behavior. The bands are relatively closer around the Fermi level compared to the other halogenated compounds, suggesting that the presence of bromine atoms tends to narrow the band gap and enhance electronic conductivity.The structure for C₁₆H₁₀I₂O₂ shows a wider band gap, implying more insulating behavior. This can be attributed to the larger atomic radius of iodine, which reduces orbital overlap and weakens electronic interactions, increasing the energy separation between bands.In C₁₆H₁₀Cl₂O₂, the band gap lies between those of the brominated and iodinated analogues, implying that chlorine has a more balanced effect. It provides moderate orbital overlap, resulting in intermediate semiconducting behavior.The band structure of C₁₆H₁₀F₂O₂ exhibits a distinct trend. Due to the high electronegativity and small atomic radius of fluorine, there is stronger localization of electrons, which leads to a more rigid band structure and a relatively wider band gap compared to Br and Cl, though slightly narrower than that of I. This suggests that fluorine substitution contributes to enhanced electronic stability but at the cost of reduced conductivity.Overall, the results clearly demonstrate that halogen substitution significantly modulates the band gap and, consequently, the electronic behavior of the C₁₆H₁₀X₂O₂ series. The calculated band gaps for the C₁₆H₁₀X₂O₂ compounds follow the trend: I , with iodine-substituted molecules exhibiting the narrowest band gap (1.63 eV), and brominated ones the widest (2.15 eV). This indicates that the incorporation of heavier halogens, particularly iodine, enhances the semiconducting behavior by narrowing the energy gap. The calculated energy band gaps for each compound are summarized in Table 2, highlighting the progressive variation across the halogen series.
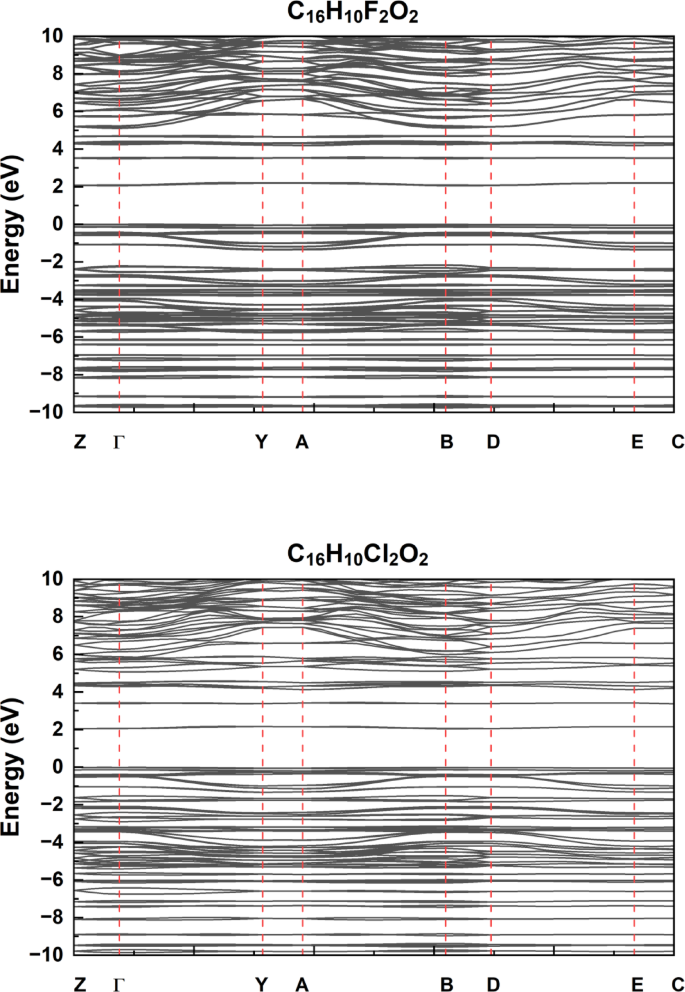
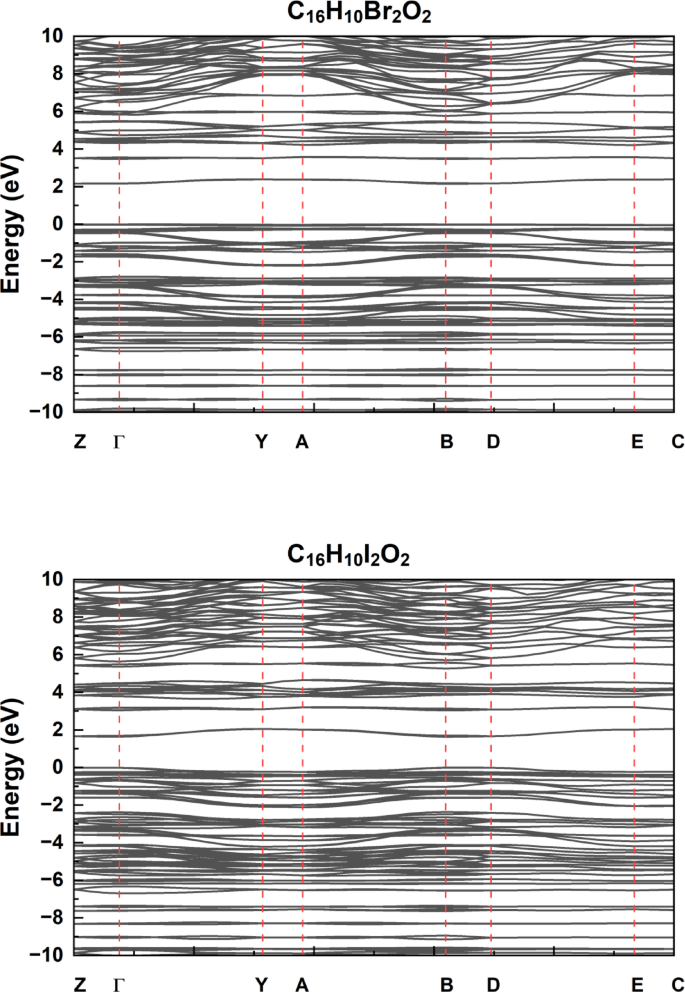
Electronic Band Structure of for the C₁₆H₁₀X₂O₂ compounds (where X = F, Cl, Iand Br): High-Symmetry Path Analysis in Reciprocal Space.
Density of state
As shown in Fig. 3, analysis of the Partial Density of States (PDOS) and Total Density of States (TDOS) curves for the C₁₆H₁₀Br₂O₂ compound reveals a distinctive distribution of electronic properties across the energy range from − 10 to + 10 eV. These curves expose a complex electronic structure where C (p) orbitals contribute predominantly to the density of states, with prominent peaks at −6, −4, and − 2 eV in the valence band region (negative energy). The contribution of Br (p) orbitals is clearly visible in Fig. 3, especially in the range from − 2 to 0 eV, while hydrogen and oxygen contributions remain relatively minor. The curves indicate the presence of an energy gap around the Fermi level (0 eV), separating the electron-occupied valence bands from the unoccupied conduction bands. In the positive energy region, a prominent peak appears at approximately + 4 eV with significant contribution from C (p) orbitals, while the TDOS curve (yellow line) continues to rise beyond + 6 eV. Comparing these results with similar C₁₆H₁₀X₂O₂ compounds (where X = F, Cl, I) suggests that halogen substitution slightly affects the density of states distribution, with differences in curve shapes and peak heights, particularly in regions close to the Fermi level. These electronic characteristics play a pivotal role in determining the physical and chemical behavior of the compound, including electrical conductivity, optical absorption, and chemical reactivity properties.The PDOS curves presented in this study show a pattern similar to that found by Lee et al. in their study of halogenated organic derivatives, where halogen p orbitals play a pivotal role in determining the properties of upper valence bands, affecting the electronic gap and electron transitions25.
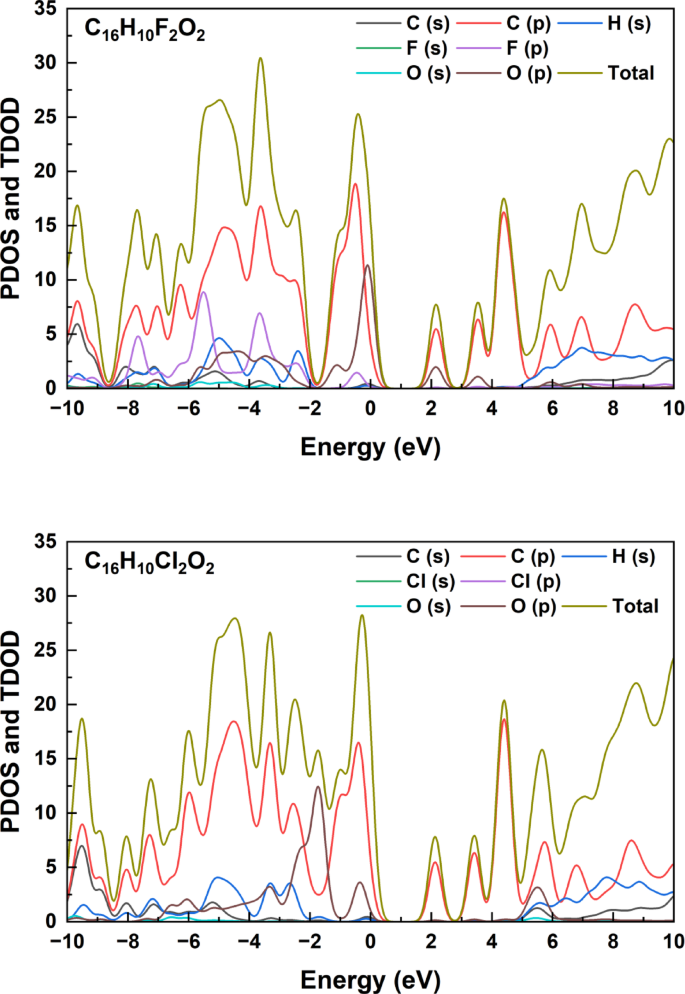
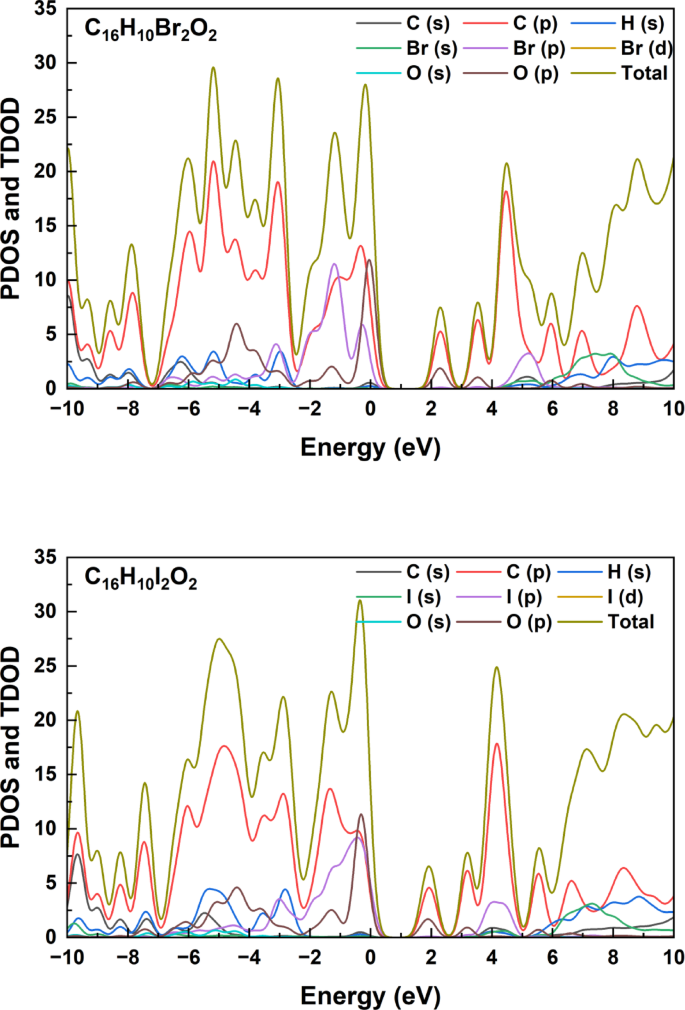
Partial Density of States (PDOS) and Total Density of States (TDOS) curves for the C₁₆H₁₀X₂O₂ compounds (where X = F, Cl, Iand Br).
Optical properties
Reflectivity
The reflectivity spectra of C₁₆H₁₀X₂O₂ compounds containing halogen atoms (X = F, Cl, Br, I), as shown in Fig. 4, display distinct optical behavior across a broad spectral range from approximately 30 nm to 1200 nm. In the short-wavelength region (30–400 nm), corresponding to the ultraviolet (UV) and vacuum ultraviolet (VUV) domains, sharp reflectivity peaks are observed for all compounds. These peaks are attributed to π → π* and n → π* electronic transitions, as well as excitations from deep valence or semi-core states. The maximum reflectivity is recorded around 50–100 nm, particularly for C₁₆H₁₀Cl₂O₂ and C₁₆H₁₀I₂O₂, suggesting a strong interaction between incident photons and localized electronic states. This behavior highlights the influence of halogen electronegativity and polarizability on the dielectric response of the materials. As the wavelength increases beyond 400 nm (visible range), reflectivity gradually decreases, consistent with a reduction in electronic excitations. The spectra begin to converge toward the upper end of the range, indicating similar optical responses among the compounds in the near-infrared (NIR) region. Among all compounds, C₁₆H₁₀Cl₂O₂ exhibits the highest reflectivity across most of the spectrum, while C₁₆H₁₀F₂O₂ shows the lowest, reinforcing the role of halogen substitution in tailoring the light–matter interaction in organic semiconductors.
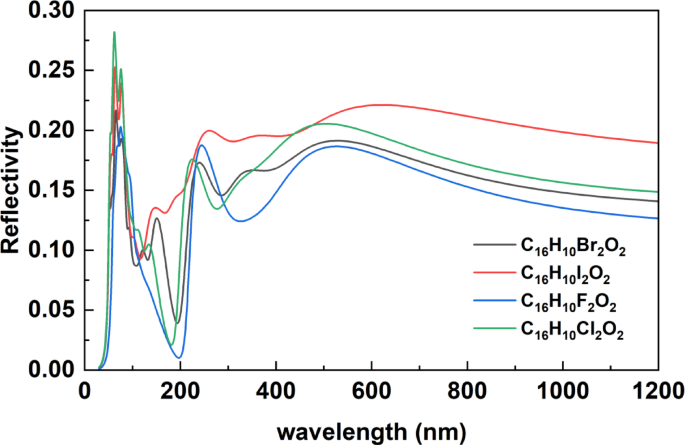
Reflectivity Spectra of Halogenated C₁₆H₁₀X₂O₂ Compounds (X = F, Cl, Br, I).
Absorption
The absorption spectra of the halogenated organic compounds C₁₆H₁₀X₂O₂ (X = F, Cl, Br, I), now plotted as a function of wavelength (nm) (Fig. 5), demonstrate clear optical trends across a broad spectral range from 0 to 1200 nm. In the short-wavelength region (30–200 nm), corresponding to the vacuum ultraviolet (VUV) and deep ultraviolet (DUV), all compounds show sharp and intense absorption peaks. These features are attributed to π → π* and n → π* electronic transitions and deep interband excitations within the valence band. The chlorinated compound (C₁₆H₁₀Cl₂O₂) exhibits the highest absorption peak, reaching nearly 280,000 units around 77.5 nm, while C₁₆H₁₀F₂O₂ shows a slightly blue-shifted maximum near 69 nm, indicating higher energy excitations. The brominated and iodinated compounds present similar spectral profiles, with broader peaks and slightly shifted absorption edges. Beyond 200 nm, all compounds show rapidly decreasing absorption, and approach near-zero values beyond 800–1000 nm, indicating negligible optical absorption in the near-infrared (NIR) region. These spectral variations clearly reflect the influence of halogen substitution on the electronic structure and light–matter interactions of the molecules. When compared to the reflectivity data, the absorption peaks generally correspond to minima or inflection points in reflectivity, highlighting the expected inverse relationship between the two properties in accordance with fundamental optical principles. The reflectivity and absorption spectra results align with the study by Yamamoto et al.26 who demonstrated that halogen substitution in organic compounds leads to systematic changes in optical properties, particularly in the energy range of 15–25 eV which is associated with core-level transitions.
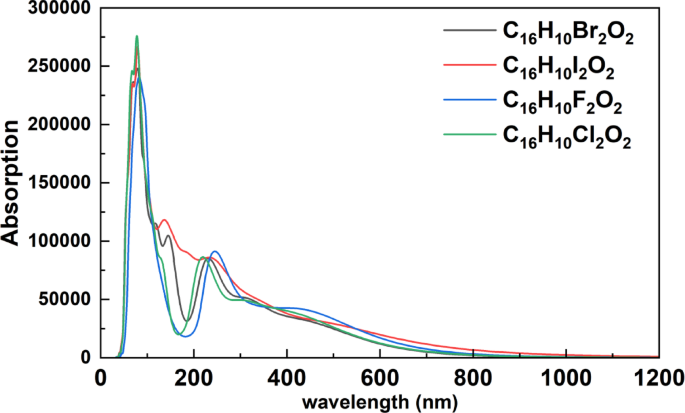
Absorption Spectra of Halogenated C₁₆H₁₀X₂O₂ Compounds (X = F, Cl, Br, I).
Refractive index
Figure 6, presents the refractive index spectra of the halogen-substituted organic compounds C₁₆H₁₀X₂O₂ (X = F, Cl, Br, I), plotted as a function of wavelength (nm) across the spectral range of 0 to 1200 nm. In the short-wavelength region (30–200 nm), which corresponds to the vacuum and deep ultraviolet, all compounds exhibit pronounced variations in refractive index. Clear peaks are observed between approximately 100–200 nm, reflecting the strong dispersion behavior due to electronic transitions in this high-energy region. As the wavelength increases toward the visible spectrum (400–700 nm), the refractive index begins to stabilize gradually. The compound C₁₆H₁₀I₂O₂ (iodine) shows the highest refractive index values in this range (~ 2.6–2.7), followed by C₁₆H₁₀Br₂O₂, C₁₆H₁₀Cl₂O₂, and finally C₁₆H₁₀F₂O₂. This trend correlates with the increasing atomic number and polarizability of the halogen atoms, where heavier halogens contribute more significantly to the material’s optical response. In the near-infrared region (700–1200 nm), the refractive index curves become nearly flat for all compounds, indicating a stable optical response and reduced dispersion as photon energy decreases. These observations are consistent with the earlier results from absorption and reflectivity spectra. The regions of high refractive index typically align with the onset of absorption or reflectivity features, supporting the underlying physical relationship described by the Kramers-Kronig relations. The systematic differences in optical response caused by halogen substitution offer valuable insights for tuning the electronic and optical properties of organic semiconductors. These findings suggest potential for the design of custom optical materials tailored for use in optoelectronic applications.
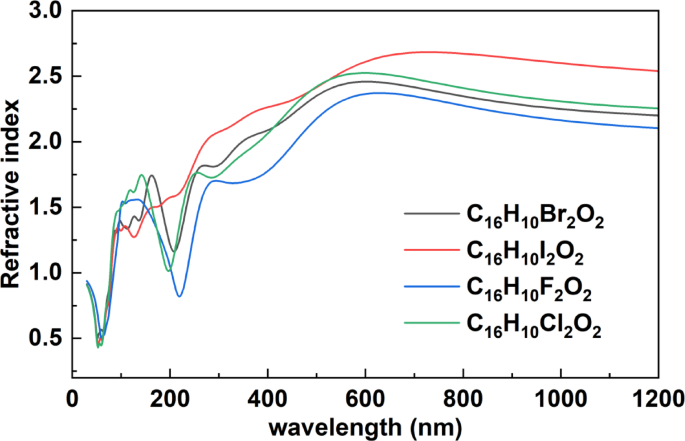
The refractive index Spectra of Halogenated C₁₆H₁₀X₂O₂ Compounds (X = F, Cl, Br, I).
Loss function
Figure 7 shows the loss function spectra of the halogenated organic compounds C₁₆H₁₀X₂O₂ (X = F, Cl, Br, I), plotted as a function of wavelength (nm) across the spectral range of 0–1200 nm. The loss function represents the energy dissipation of fast electrons interacting with the material and provides insight into plasmonic resonances and electronic excitation mechanisms. All compounds exhibit a dominant peak at short wavelengths (~ 50–60 nm), which corresponds to high-energy plasmonic excitations typically occurring when the real part of the dielectric function approaches zero. Among the compounds, C₁₆H₁₀Cl₂O₂ shows the most intense peak (~ 3.4), followed by C₁₆H₁₀I₂O₂, C₁₆H₁₀F₂O₂, and C₁₆H₁₀Br₂O₂. These variations confirm the strong influence of the halogen atom on collective electronic behavior. A secondary feature appears around 150–200 nm, most clearly in C₁₆H₁₀F₂O₂, which indicates the presence of low-energy excitation modes such as interband transitions or possible surface plasmon activity. Beyond 300 nm, the loss function values decrease sharply and remain near zero throughout the visible and near-infrared regions, indicating minimal energy dissipation from collective excitations in these longer-wavelength ranges. These results are consistent with previously analyzed refractive index, reflectivity, and absorption spectra, reinforcing the relationship described by the Kramers-Kronig relations. Notably, the main plasmon resonance near 50–60 nm corresponds to wavelengths where the refractive index reaches its minimum and absorption is reduced, supporting the identification of strong collective electronic oscillations. The differences observed between halogenated compounds reflect the role of atomic polarizability and electronic structure in modulating energy loss behavior. The chlorinated derivative demonstrates the most prominent collective excitation, suggesting enhanced light-matter coupling due to the nature of the Cl atom. This wavelength-based analysis offers a comprehensive view of energy loss dynamics in these compounds and supports their potential for applications in plasmonic sensors, nanophotonics, and optical energy management, where control of energy dissipation is critical. The notable differences in the loss function spectra among different halogenated compounds align with findings by Zhang et al.27 who demonstrated that the plasmonic response of organic compounds is significantly affected by the type of halogen substituent, with systematic changes in the intensity and position of collective excitation peaks.
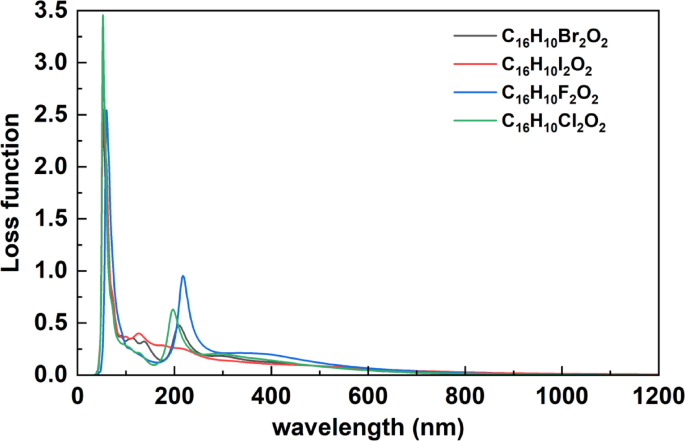
Loss function Spectra of Halogenated C₁₆H₁₀X₂O₂ Compounds(X = F, Cl, Br, I).

