In the previous post[1] I mooted the possibility that a high energy form of the dimer of nitric oxide 1 might nonetheless be able to be detected using suitable traps (such as hydrogenation or cycloaddition). However, an interesting alternative is that this species could be trapped by nitric oxide itself. According to [2] in an article entitled “Decomposition of nitric oxide at elevated pressures” the rate of this termolecular reaction 3NO → N2O + NO2 are said to obey third order kinetics. One plausible mechanism for this process is shown below.
So the question now arises as to whether this process would always intervene to prevent any trapping of species 1. Time for some calculated energies.[3] The reaction occurs in two steps, TS1, forming Int, which then gives the product via TS2. The combined IRC for these processes is shown below,† and the total energy barrier from 1 + NO for this process is small (~7 kcal/mol).
The NN bond length along the course of these two steps is shown below,† indicting that it starts and ends with an ~N≡N bond, as implied in the scheme above.
The dipole moment response is shown below.†
Animation for the IRC for TS1 is shown below:
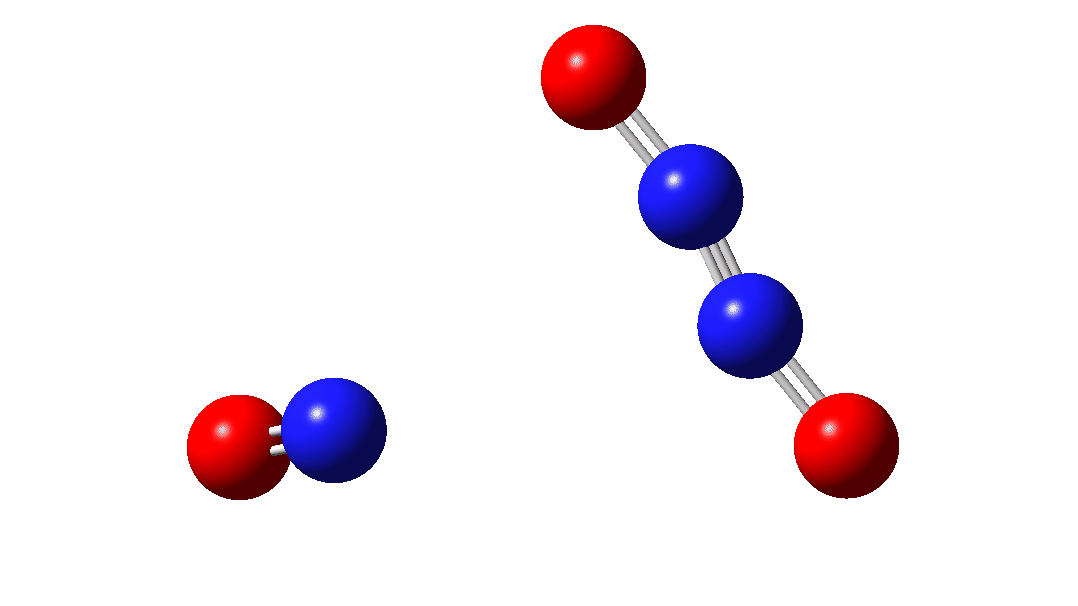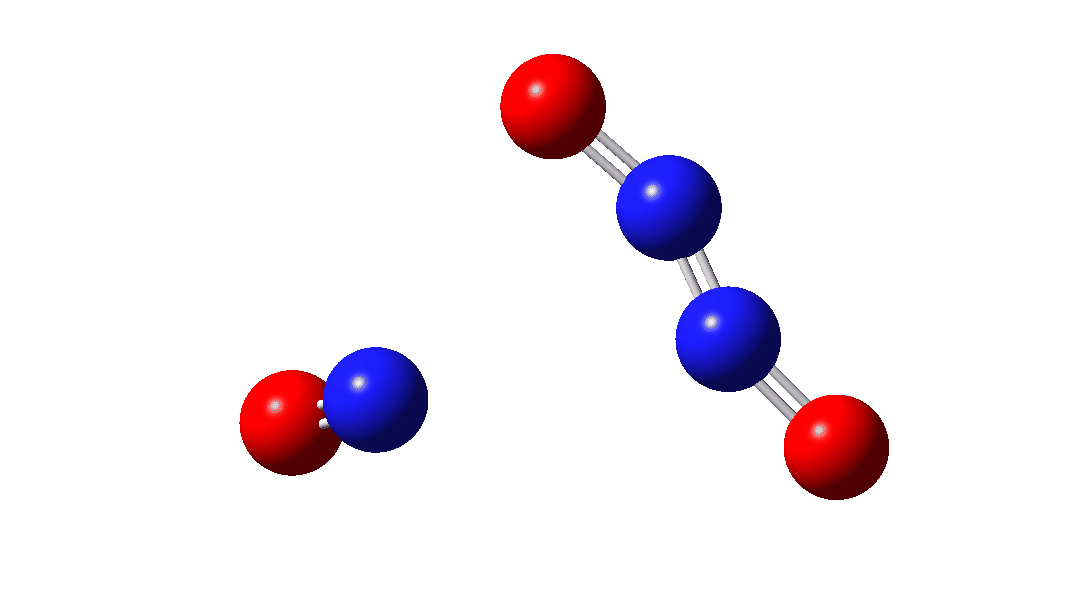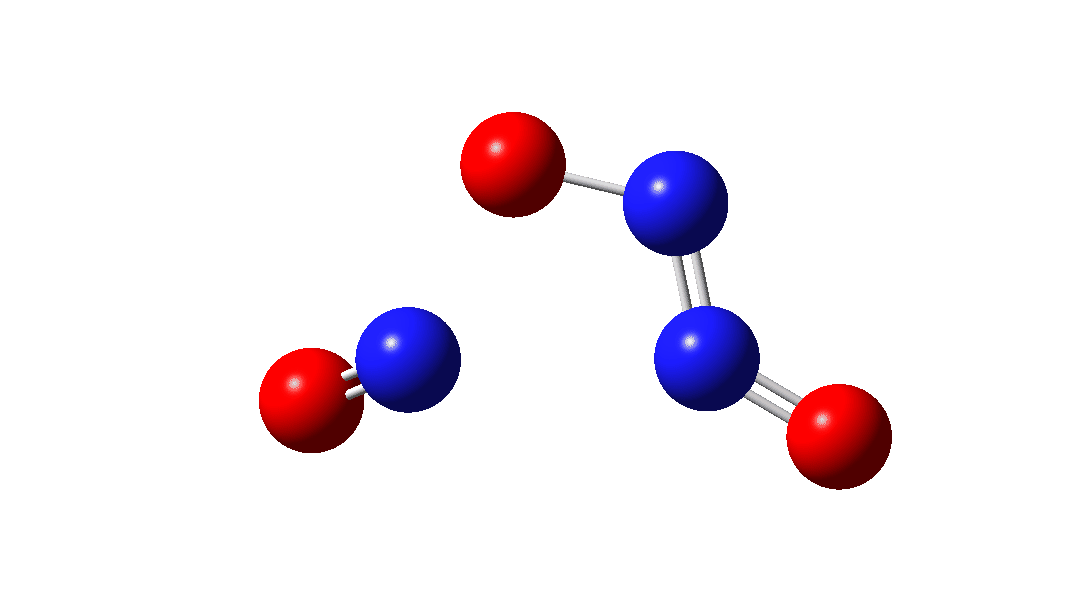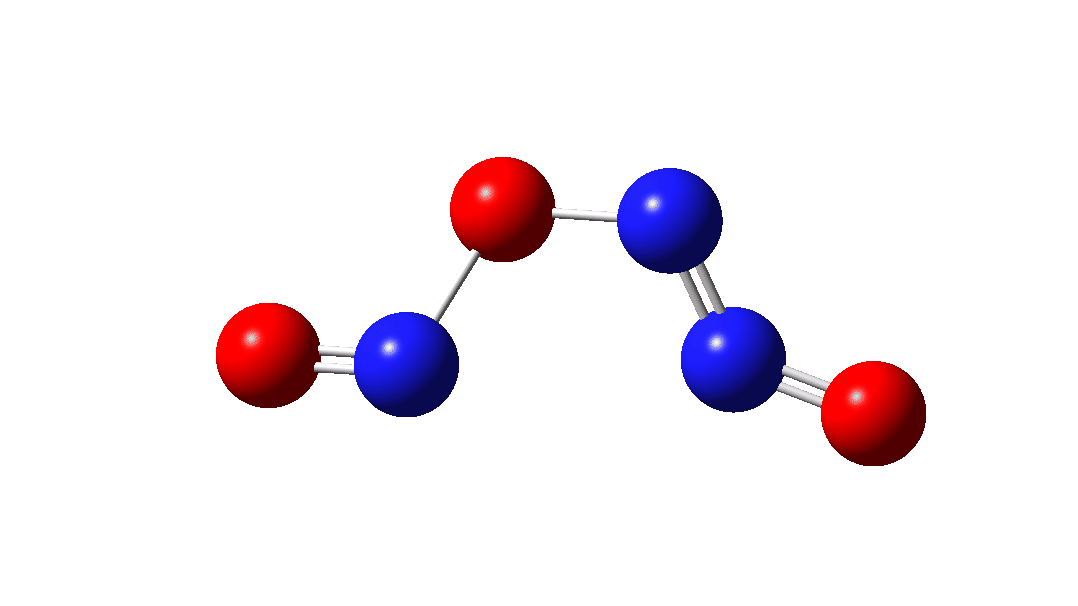
The intermediate, N3O3 has a spin density covering all six atoms (click on image below to see interactive 3D model) so is difficult to represent by e.g. a single valence bond structure as shown above.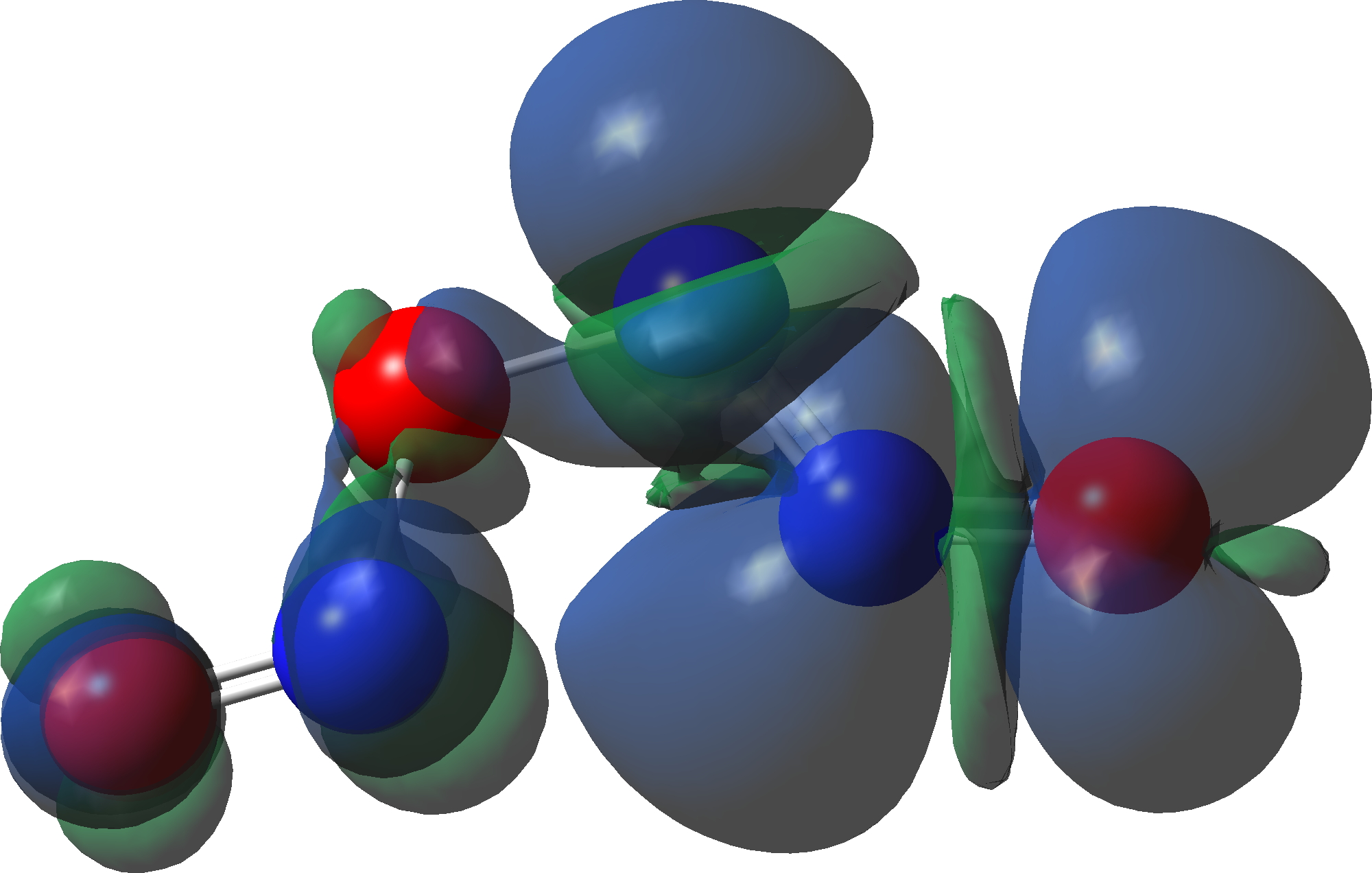
The IRC for TS2 is animated below:
The high value for ΔG‡ ~39.5 kcal/mol arises in large part because of the loss of entropy in reducing three molecules to one at the transition states – and can be compared with e.g. the value of ΔG‡ 31.0 kcal/mol previously quoted for the bimolecular dimerisation of two molecules of NO.[4]
The story is not quite over yet, since other conformations for this reaction are still being explored. Currently it appears there is only a small free energy window between forming species 1 and it reacting further in the manner 3NO → N2O + NO2 as shown above, so it seems unlikely at this stage that 1 could be easily detected.
† The slight discontinuity visible at ~IRC +1 is due to a conformational change between the end point of the IRC for TS1 and the start point for TS2.
DOI: 10.59350/rzepa.29665
References
- H. Rzepa, “Hydrogenating the even more mysterious N≡N triple bond in a nitric oxide dimer.”, 2025.
- T. Melia, “Decomposition of nitric oxide at elevated pressures”, Journal of Inorganic and Nuclear Chemistry, vol. 27, pp. 95-98, 1965.
- H. Rzepa, “Alternative reactions of the N≡N triple bond in a nitric oxide dimer.”, 2025.
- H. Rzepa, “The even more mysterious N≡N triple bond in a nitric oxide dimer.”, 2025.
Related
You can leave a response, or trackback from your own site.

1 Comment
https://shorturl.fm/CR7m4