Affirmation of the structures of the prepared polymers
IR FT-IR spectra of the prepared polymers; TPO and NTPO were illustrated in Fig. 2. The peaks were analysed in Table 2, where the peaks of esters, C=O at 1730–1750 cm−1, C–H stretches at 2800–2900 cm−1, C–H bending at 1160–1169 cm−1 and presence a peak at 465 cm−1 at refers to the NB-OL bond with the polymer.
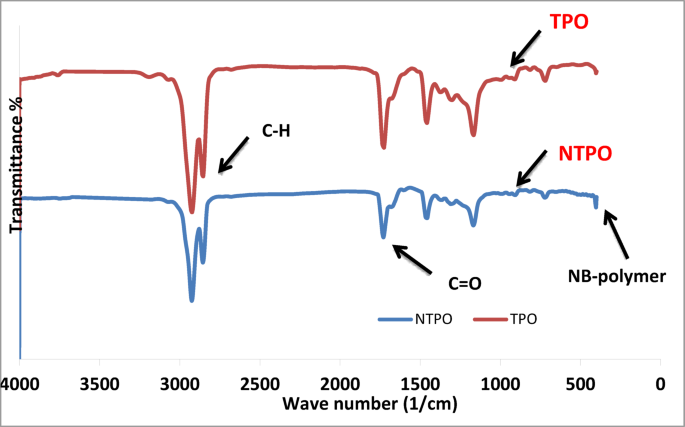
FT-IR spectrum of terpolymer (TPO) and terpolymer nanobentonite.
1H NMR The 1H NMR spectra of the synthesized polymers; TPO and NTPO (Fig. 3a, b) exhibited characteristic proton signals (δ, ppm) at 0.8–0.9 ppm (–CH3), 1.4 ppm (–CH2–CH3), 2.3–2.5 ppm (–CH2– C=O), and 3.5–3.9 ppm (–CH2–O–), confirming the expected polymer structure and successful Synthesis. As mentioned in Table 2.
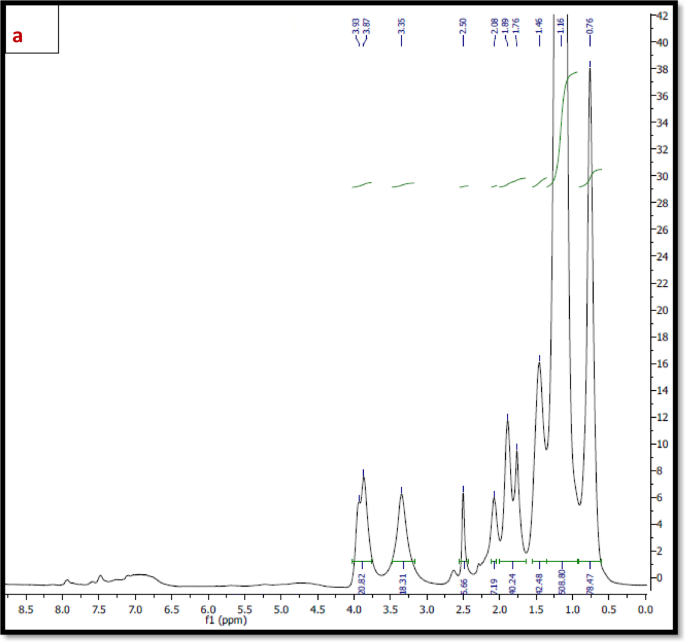
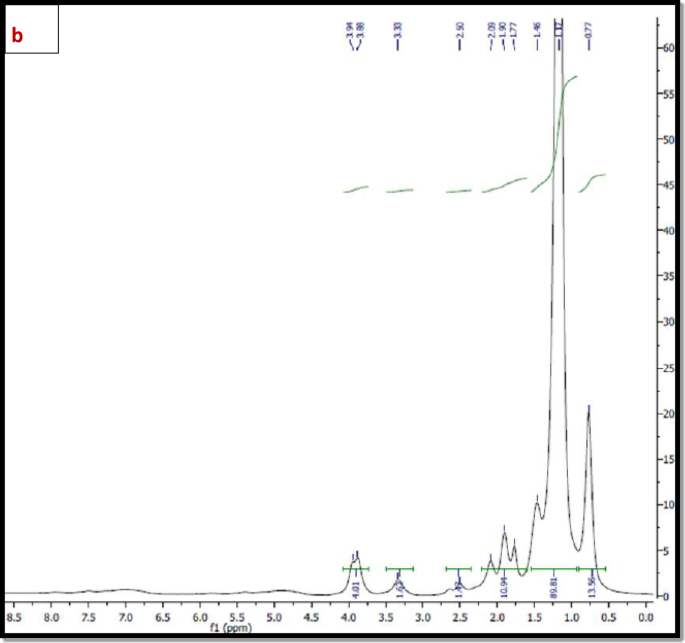
(a) 1H NMR spectrum of Terpolymer (TPO), (b) 1H NMR spectrum of nano-bentonite polymer NTPO.
HRTEM The HRTEM analysis image of the polymer nanocomposite (NTPO) is shown in Fig. 4a. The TEM image clearly shows the distribution of nano-bentonite particles within the polymer matrix, where the large dark regions represent the bulk polymer phase and the bright white dots represent the embedded nano-bentonite. This distribution throughout the polymer confirms the successful Synthesis of the NTPO nanocomposite.
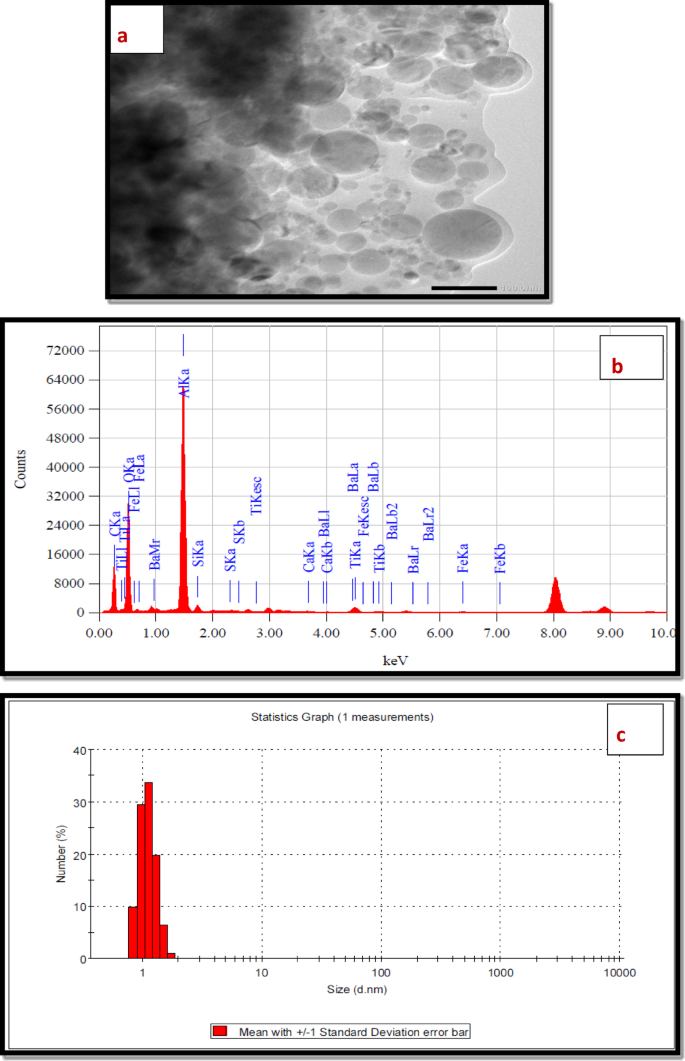
(a) TEM, (b) EDX and (c) DLS of the prepared nano-bentonite polymer (NTPO).
EDX spectra The EDX analysis of the nanocomposite showed intense carbon and oxygen peaks from the polymer matrix, along with distinct signals for silicon, aluminum, magnesium, potassium, and calcium – elements characteristic of nano-bentonite. These findings confirm the successful incorporation and dispersion of the nano-bentonite particles within the terpolymer structure, as evidenced in Fig. 4b. The quantitative analyses of EDX were mentioned in Table 3.
DLS The DLS analysis of the NTPO nanocomposite sample revealed a narrow size distribution with particles below 10 nm, as visible in Fig. 4c. The measurement scale clearly shows particles around 2 nm, confirming the successful formation of the nanohybrid material. These results demonstrate the effective production of the polymer nanocomposite with well-controlled nanoparticle dimensions. The analysis of the Figure was discussed in Table 4.
TGA and DSC of TPO and NTPO The thermal stability of the prepared terpolymer (TPO) and its nanocomposite (NTPO) was investigated by TGA, as presented in Fig. 5. Both materials exhibited a single-step major weight loss, indicating a relatively homogeneous degradation mechanism. For both TPO and NTPO, the initial thermal decomposition onset was observed around 240–260 °C, which corresponds to the breakdown of side chains and weak polymeric linkages. The major degradation stage occurred between 300 and 440 °C, representing backbone scission and volatilization of the organic matrix. Beyond 450 °C, only a minimal residue remained, confirming almost complete decomposition of the organic fraction. A comparison between the two curves shows that NTPO displays slightly improved thermal resistance in the early stages of degradation, with a slower weight-loss rate compared to pure TPO. This behavior can be attributed to the presence of nanobentonite, which provides a barrier effect, delaying thermal diffusion and enhancing polymer stability. Overall, both TPO and NTPO exhibit thermal stability up to 240 °C before degradation begins, which is significantly higher than the operating temperatures of diesel fuels. This confirms that the synthesized additives are stable under practical fuel application conditions and suitable for use as pour point depressants.
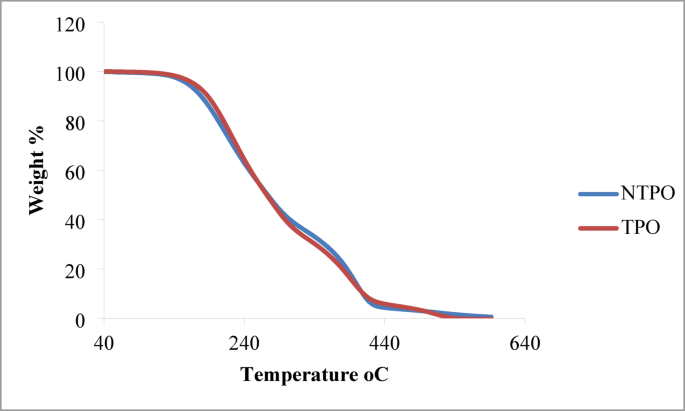
Thermal gravimetric analysis (TGA) curves of TPO and NTPO indicating their thermal stability profiles.
The thermal behavior of the synthesized polymers was evaluated using DSC, as shown in Fig. 6a, b. For the TPO, a series of minor transitions were observed below 0 °C, which can be attributed to side-chain relaxations or partial crystallization of alkyl acrylate moieties. A distinct transition in the 0–20 °C range corresponds to the glass transition (Tg) and possible melting of short-range ordered domains. Beyond this region, the curve displayed a gradual endothermic decline up to 300 °C, which is associated with the softening of the backbone and the onset of degradation. The absence of a sharp decomposition peak indicates good thermal stability. In contrast, the NTPO exhibited more intense exothermic events around 0 °C, suggesting a higher degree of side-chain crystallization and stronger ordering of the polymer segments. The deeper transitions also reflect a higher enthalpy change, consistent with increased crystallinity compared to the first polymer. Similar to the TPO, a broad endothermic trend extending from 100 to 300 °C was observed, indicating that both materials are thermally stable in this range. Comparatively, the TPO exhibits lower crystallinity and a more amorphous character, which can impart improved flexibility and low-temperature processability. The NTPO, with its higher crystallinity, may provide more structural rigidity but reduced flexibility at sub-ambient conditions. Nevertheless, both polymers demonstrate thermal stability with degradation onset above 250–300 °C, which is sufficient for their intended application as diesel fuel pour point depressant (PPD) additives.
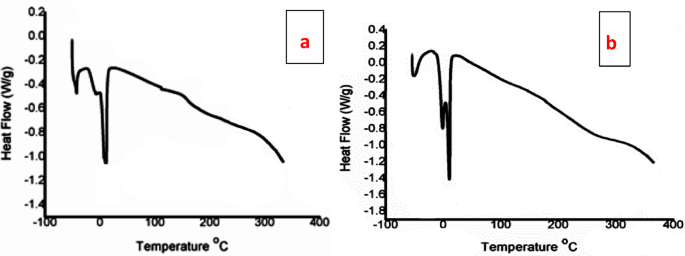
DSC thermograms of the (a) TPO and (b) NTPO.
Impact of TPO and NTPO as PPDs
The pour point represents the minimum temperature at which diesel fuel retains its ability to flow under gravitational force. High pour points in waxy diesel fuels create challenges during cold-weather transportation due to paraffin wax crystallization. Pour point depressants work by altering wax crystal morphology, reducing their size and cohesion, which improves low-temperature fluidity62,63,64.
The pour point temperatures of diesel fuel were measured after treatment with varying concentrations (1000, 3000, 5000, 10,000, and 15,000 mg/L) of TPO and NTPO additives, and measured according to ASTM D97 (measure the temperature every 3 °C until the oil stops flowing), then replicate the test 3 times to ensure the results. The experimental data, summarized in and illustrated in Fig. 7, revealed that NTPO exhibited greater effectiveness than TPO in reducing the pour point of diesel fuel. In case of treated diesel fuel, as the concentration of terpolymer increased, the treated diesel fuel oil’s pour point values gradually decreased. Therefore, it was found that utilizing 10,000 mg/L NTPO had the greatest potency for lowering the treated diesel fuel oil’s pour point from − 3 to – 33 °C. It was found that treatment with TPO resulted in a depression to − 27 °C. The higher concentration (15,000 mg/L) at TPO additive lead to slowly increase in the PP (− 24 °C) this may be attributed to the agglomeration of the polymer that hinder the dispersant of the wax but with using NTPO show the same results of 10,000 mg/L (− 33 °C) this is from to the effect of the nanobentonite that help the polymer to be more dispersed.
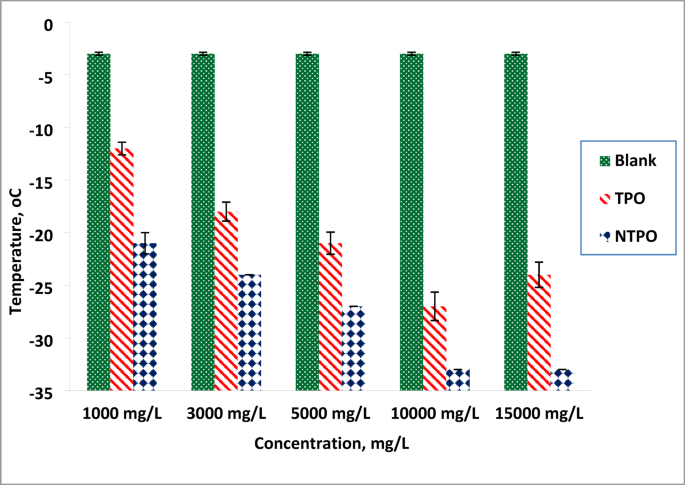
Variations in pour point temperatures of the treated and untreated diesel fuel.
The enhanced performance at higher concentrations occurs through co-crystallization with paraffin wax, which modifies crystal morphology to prevent aggregation. At 10,000 mg/L, the additive effectively alters crystal structure, creating a barrier against three-dimensional wax network formation. In contrast, lower concentrations (65. This partial action at lower doses only slows lateral crystal growth without completely preventing wax formation18.
These results establish 10,000 mg/L NTPO as the most effective concentration for pour point depression, where complete crystal modification occurs to maximize low-temperature flow improvement in diesel fuels. NTPO’s superior efficiency stems from nanoparticle adsorption on wax crystals, which more effectively disrupts their 3D lattice formation. This nanoscale interaction enables fuel flow at significantly reduced temperatures, demonstrating the nanocomposite’s potential as a high-performance pour point depressant66.
The cloud point (CP) of the base diesel and samples treated with TPO and NTPO are shown in Table 5. The untreated diesel (blank) exhibited a CP of 6 °C. Addition of TPO reduced CP progressively to 3 °C at 10,000–15,000 mg L−1 (1.0–1.5 wt%), indicating improved resistance to wax appearance. The NTPO nanocomposite demonstrated superior performance: at the same dosages, CP fell to 2 °C, confirming that incorporation of nanobentonite enhances additive efficiency. These trends support the proposed mechanism whereby the terpolymer (long alkyl side chains) together with nanobentonite interferes with wax nucleation and growth, delaying the onset of visible crystallization (lower CP). The cloud point (CP) data show a clear reduction with increasing additive concentration for both TPO and NTPO, confirming their efficiency as pour point depressants. The low standard deviations (± 0.5 °C) across all measurements indicate high reproducibility and reliability of the experimental results, which enhances the credibility of the observed trends.
The wax appearance temperature (WAT) The viscosity–temperature data were plotted, and the wax appearance temperature (WAT) was determined from the deviation point in the curves, as illustrated in Fig. 8. The wax appearance temperature (WAT) of the blank diesel sample was observed at 10 °C, indicating that wax crystals start to form at relatively high temperatures, which adversely affects the fuel flow properties under cold conditions. Upon the addition of TPO, the WAT significantly decreased to 3 °C, representing a reduction of about 7 °C compared to the blank. This pronounced shift confirms the efficiency of TPO in delaying the onset of wax crystallization by interfering with wax crystal nucleation and growth. Notably, the NTPO further reduced the WAT to − 5 °C, achieving an overall reduction of approximately 15 °C compared to the untreated fuel. This superior performance can be attributed to the synergistic effect of the nano-bentonite incorporated within the polymer matrix, which provides additional interaction sites with paraffin molecules and hinders crystal agglomeration. These findings clearly demonstrate that NTPO exhibits enhanced pour point depressant activity compared to TPO alone.
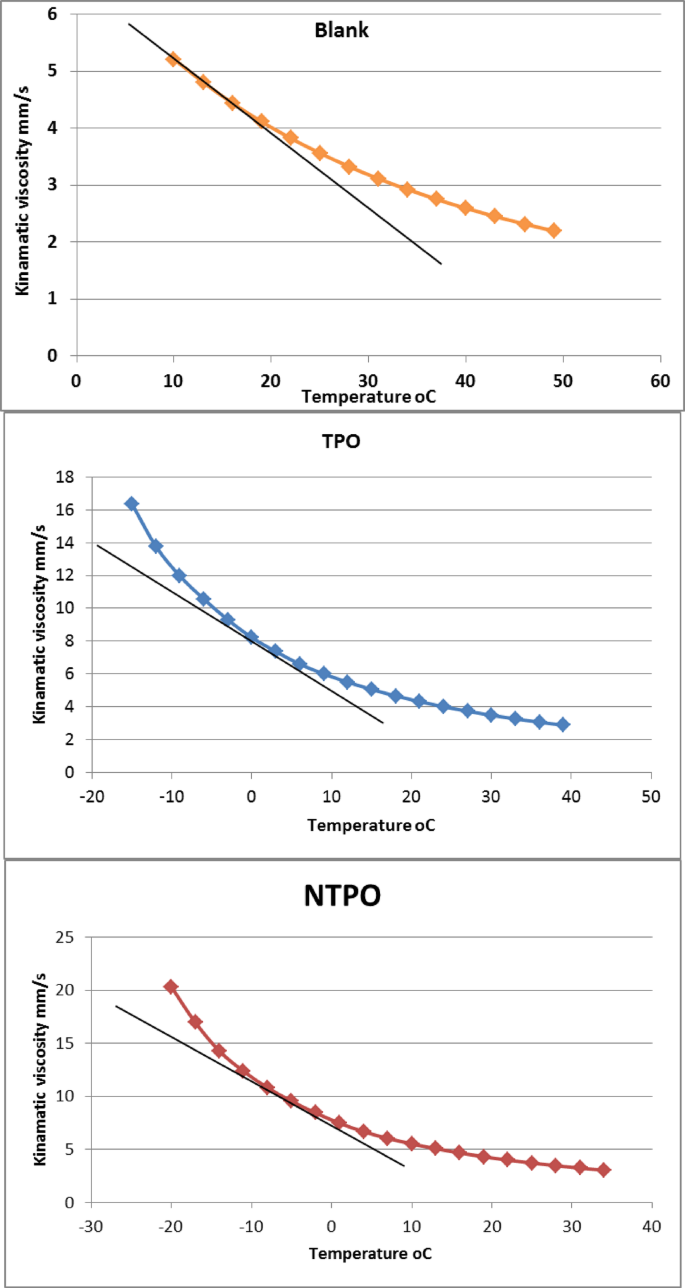
Viscosity–temperature curves of blank, TPO-treated, and NTPO-treated diesel samples.
The previous commercial studies were compared with this study and tabulated in Table 6. The Table shows that conventional commercial PPDs such as EVA and PMA typically achieve 12–25 °C pour point reduction at dosages (0.1–0.5 wt%). In this study, the PP reduction achieved was 18–30 °C with the dose of the TPO/NTPO additives (0.1–1.0 wt%), the deduction at 0.1 wt% was 18 °C, and this is superior compared to the commercial to achieve this reduction at a relatively low concentration. Also, TPO/NTPO still depresses the temperature until it reaches the dose of 1 wt%. The achieved pour point depression was comparable to or better than some commercial benchmarks. This indicates potential practical utility, although optimization of synthesis and dosage is needed to reduce additive concentration and improve cost-effectiveness.
Although the additives demonstrated effective pour point depression in fresh diesel samples, future studies should investigate durability under real storage and thermal conditions, including sedimentation, storage stability, and thermal aging, to establish industrial feasibility.
Evaluation of TPO and NTPO as viscosity index improvers (VII) in diesel fuel
The viscosity of diesel fuel is one of its most critical properties. As the temperature rises, the solvation power of diesel fuel increases, leading to a reduction in viscosity. This occurs because polymer molecules swell, increasing their hydrodynamic volume. Although this swelling compensates for the viscosity drop, the overall viscosity of the fuel still decreases67.
Kinematic viscosity measurements were conducted on diesel fuel both with and without the addition of TPO and NTPO at a concentration of 10,000 mg/L, tested at 40 °C and 100 °C. The results, summarized in Table 7 and Fig. 9, demonstrate that both TPO and NTPO enhance the viscosity index (VI) of diesel fuel.
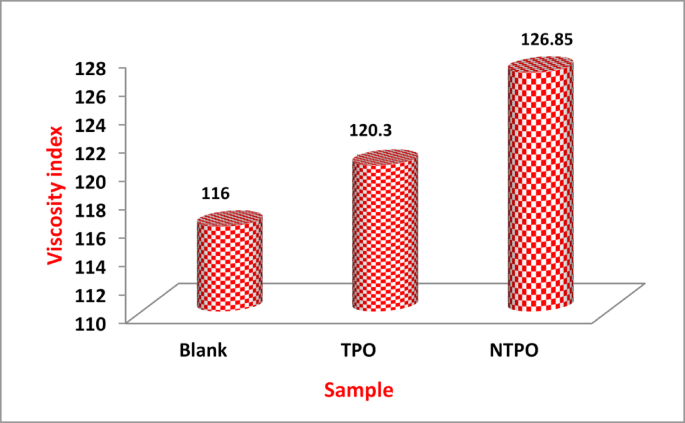
Variations in viscosity index of the treated and untreated diesel fuel.
When comparing the effectiveness of TPO and NTPO as viscosity index improvers (VII), the untreated diesel fuel had a VI of 116. However, treatment with either additive significantly improved the VI, with NTPO (126.85) outperforming TPO (120.3).
This study highlights the potential of TPO and NTPO as effective additives for improving the viscosity index of diesel fuel, with NTPO showing superior performance. Also, by comparison of this study with commercial diesel fuel as VI-improver, that summarized in Table 8, referring to the superiority of this work as VII.
Rheological behavior and flow characteristics of diesel fuel oil
The rheological properties of diesel fuel exhibit shear-dependent behavior during cooling, where applied shear stress inhibits paraffin crystallization and maintains flow-ability. Below a critical shear rate threshold, progressive wax precipitation increases apparent viscosity and induces gel formation, potentially leading to pipeline flow cessation. When subjected to shear, interactions between polymeric molecules and wax crystals lead to crystal fragmentation, producing particles with varying sizes, shapes, and rheological properties. Therefore, addressing these flow challenges—particularly in cold environments—requires a thorough understanding of fuel oil rheology. The flow behavior is governed by compositional factors (wax type, quantity, and nature), crystallization behavior, and operational conditions, including applied shear rate, shearing duration, temperature, and cooling rate67.
This study conducted viscosity measurements for untreated and treated diesel fuel at 20 °C, 30 °C, and 40 °C, maintaining a consistent polymeric additive concentration of 10,000 mg/L. Figures 10, 11 and 12 illustrate variations in shear stress (Figs. 10, 11 and 12a) and viscosity (Figs. 10, 11 and 12a, b) as functions of shear rate. The experimental data demonstrate a proportional rise in shear stress as shear rate increases, consistent at all tested temperatures. The untreated fuel exhibits non-Newtonian, yield-pseudoplastic behavior, characterized by an abrupt rise in shear stress at higher shear rates. In contrast, treated diesel demonstrates a linear viscosity reduction with increasing shear rate, eventually stabilizing at elevated shear rates. Notably, NTPO outperforms TPO as a flow improver (FI), aligning with prior findings 68.
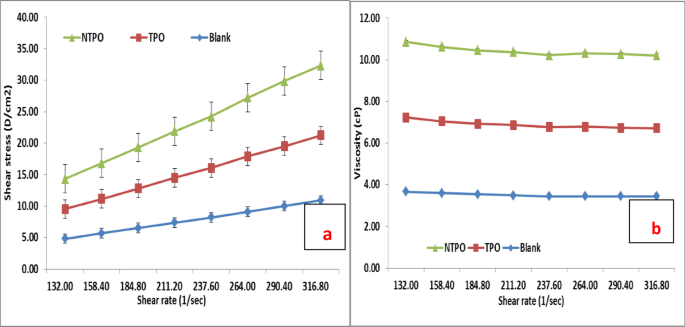
Shear rate versus (a) Shear stress and (b) Viscosity of treated and untreated diesel fuel at 20 °C.
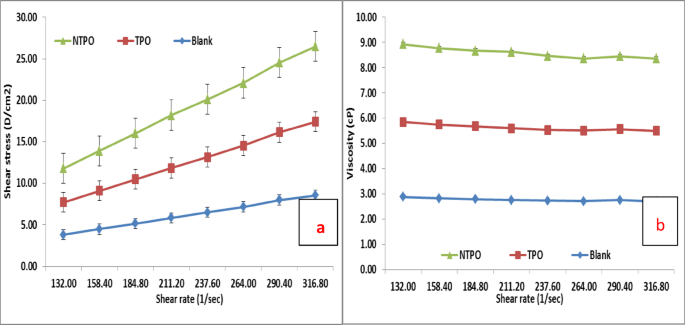
Shear rate versus (a) Shear stress and (b) Viscosity of treated and untreated diesel fuel at 30 °C.
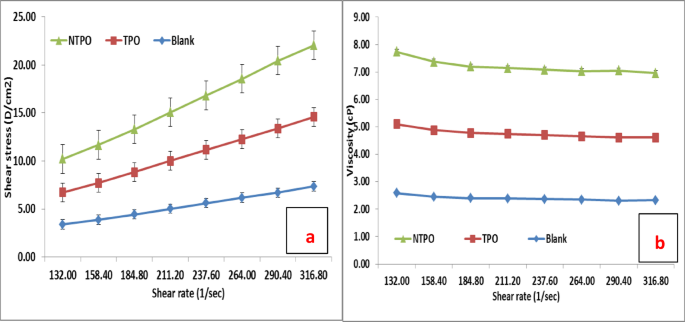
Shear rate versus (a) Shear stress and (b) Viscosity of treated and untreated diesel fuel at 40 °C.
At temperatures near the pour point and under low shear, wax crystals undergo partial disruption due to applied shear energy. Higher shear rates enhance energy dissipation, overcoming yield stress and initiating flow. Agglomerated structures contract under intense shear, releasing trapped continuous phase components and reducing effective dispersed phase concentration, thereby lowering viscosity. This process continues until most agglomerates disintegrate to their primary size, explaining the non-Newtonian behavior of the oil systems68,69.
Elevating the temperature from 20 to 40 °C (20 °C, 30 °C, and 40 °C) reduces both shear stress and viscosity in treated diesel. Higher temperatures amplify interactions between polymeric additives and fuel, expanding the hydrodynamic volume of polymers and increasing their effective volume fraction. Consequently, viscosity diminishes. Additionally, thermal motion weakens paraffin particle networks, further contributing to reduced viscosity. These insights underscore the critical roles of shear history and temperature in optimizing diesel fuel flow properties.
Table 9 summarizes the rheological parameters of untreated diesel and diesel treated with TPO and NTPO additives at different temperatures (20, 30, and 40 °C). For the blank diesel sample, the apparent viscosity decreases consistently with increasing temperature (from 32.8 cP at 20 °C to 21.5 cP at 40 °C), which is the expected behavior for petroleum-based fuels. The yield stress values for blank diesel are low (
All rheological measurements were performed in triplicate, and the results showed high reproducibility with minor deviations, as reflected in the error bars and standard deviation in the Table. This confirms the reliability of the observed shear stress–shear rate and viscosity trends.
Effect of TPO and NTPO on ash content
The ash content analysis provides important insight into the effect of additive incorporation on the combustion characteristics of diesel fuel. Table 10 summarizes the ash content of pure diesel fuel and that treated with TPO and NTPO. The blank diesel fuel exhibited negligible ash content (0.04%), confirming its clean-burning nature. After the addition of TPO, only a small change was detected (0.046%), which can be attributed to the organic polymeric structure of the additive. However, in the case of NTPO, the ash content increased slowly and noticeably (0.048%) due to the presence of nano-bentonite, an incombustible mineral filler. Although this increase remains within acceptable fuel specification limits, it reflects the partial contribution of inorganic residues after combustion. This outcome emphasizes the importance of balancing the advantages of wax crystal growth inhibition with the potential side effect of higher ash content in nanocomposite-based additives.
Effect of additive composition on wax crystal morphology in diesel fuel
Microscopic image analysis corroborates conventional flow property assessments by examining wax crystallization patterns in untreated and treated diesel fuel. This technique was employed to evaluate the performance of synthesized bifunctional polymeric additives (designed to function as combined pour point depressants and wax inhibitors) based on their impact on wax crystallization behavior.
Pour point depressants work by adsorption on the surface of wax crystals. The resulting surface layer of the pour point depressants prevents the development of wax crystals and their ability to absorb oil and form gels17. Photomicrographic analysis affirms other standard flow experiments, which assess the pour point depressants of treated/untreated diesel oil through the crystallization behavior of wax.
Figure 13a–c presents comparative photomicrographs of untreated diesel fuel versus samples treated with TPO and NTPO additives. Untreated fuel (Fig. 13a) exhibited large, well-defined paraffin crystal aggregates with an average size of 25.5 ± 2.7 µm, indicating uncontrolled wax precipitation. In contrast, treated samples (Fig. 13b, c) displayed significantly smaller and more uniformly dispersed crystalline particles, demonstrating enhanced paraffin dispersion. Upon addition of TPO, the wax crystals became significantly smaller and more dispersed, with an average size of 3.08 ± 0.30 µm. For the NTPO sample, the wax crystals were further reduced to nanoscale dimensions (0.79 ± 0.13 µm), indicating strong inhibition of wax growth. The substantial reduction in crystal size reflects the efficiency of the prepared additives in modifying crystal morphology and suppressing aggregation, thereby enhancing the cold flow properties of diesel fuel.
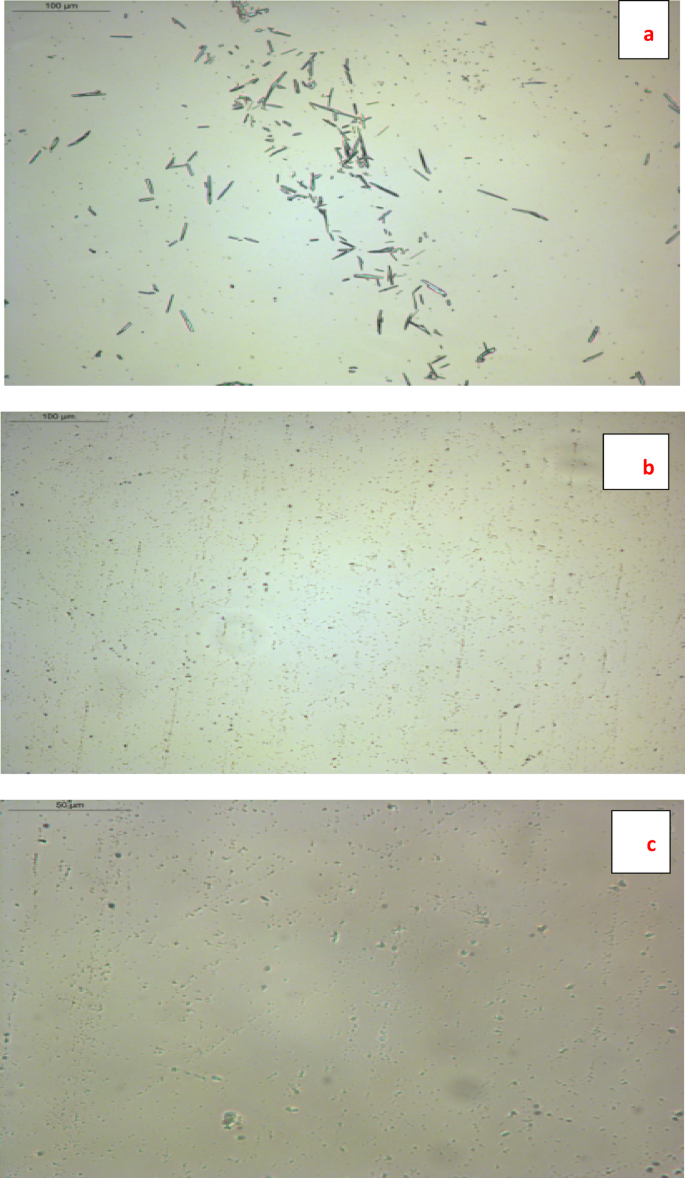
Polarized optical microscopy image (POM) of the wax of diesel fuel samples with Magnification: 200 × (a) blank with scale bar = 100 µm, (b) TPO with scale bar = 100 µm and (c) NTPO with scale bar = 50 µm.
The microscopic evidence reveals that both TPO and NTPO additives effectively modify crystallization behavior by creating multiple nucleation sites. This multi-point attachment mechanism disrupts paraffin crystal development, resulting in finer particulate distribution and consequent reduction in the fuel’s pour point. The improved dispersion characteristics confirm these additives’ efficacy in controlling wax-related flow problems in diesel fuels.
Proposed mechanism of wax deposition prevention
Figure 14 presents the proposed mechanism for how TPO and NTPO additives enhance diesel fuel’s cold flow properties. In untreated diesel, decreasing temperatures cause n-alkanes to precipitate as wax crystals. These crystals develop into large, disk-shaped structures that interconnect to form extensive three-dimensional networks. This crystalline framework traps liquid fuel molecules, significantly reducing fluidity. The additives (TPO and NTPO) modify this process by co-crystallizing with wax crystals, altering their crystallization behavior and disrupting their normal growth patterns.
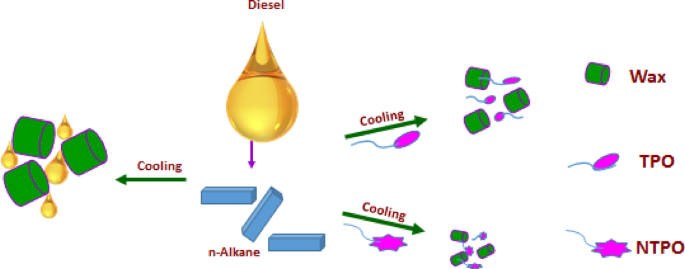
An inferred mechanism for TPO and NTPO improving the cold flow properties of diesel fuel.
The chemical additives play a crucial role in inhibiting wax crystal growth and promoting the formation of smaller crystals with high surface-to-volume ratios. A Terpolymer that contains long chains present in different three monomers used; hexadecyl acrylate, octolyate and α-olefins adsorbed on the wax molecules (paraffin) and act as the first nuclei instead of the nucleation of the wax itself. The nuclei formed repulsed from the other free wax molecule through Van der Waals forces with the aid of the polar groups in the monomers. The repulsion of wax molecules and the nuclei delays and alters the lattice formation. The delay in the formation of the crystal lattice is highly pronounced in the terpolymer nanocomposite because of the inorganic nanobentonite that helps in the formation of another Site for repulsion. Wax crystallization is typically inhibited or delayed through various mechanisms involving nucleation interference, co-crystallization, or adsorption of these additives onto nascent crystals, preventing their aggregation70. An alternative theory suggests that surface modification phenomena and thermodynamic solubility work synergistically to enhance flow properties in diesel fuel treated with polymeric additives71,72. In the present work, we assume that the prepared additives adsorb onto wax crystals via Van der Waals forces and organize themselves to restrict crystal growth through steric hindrance, as illustrated in Fig. 14.
The mechanism of cold-flow improvement can be directly correlated with the shifts in WAT, CP, and PPT. For the untreated diesel, wax crystals start to appear at relatively high temperatures (WAT = 10 °C, CP = 6 °C), leading to poor flowability at sub-ambient conditions (PPT = − 3 °C). The addition of TPO significantly delayed the onset of wax crystallization (WAT = 3 °C, CP = 3 °C, PPT = − 27 °C), indicating that the polymer molecules interact with paraffin chains and interfere with crystal nucleation and growth. A further improvement was observed with NTPO (WAT = − 5 °C, CP = 2 °C, PPT = − 33 °C), which confirms the synergistic role of nano-bentonite. The nanoparticles likely provide additional heterogeneous sites that disturb the regular packing of paraffin molecules, restrict crystal size, and prevent large-scale agglomeration. This dual effect of polymer adsorption and nanoparticle disruption explains the superior cold-flow performance of NTPO compared to TPO alone.

